Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.107 n.7 Pretoria Jul. 2017
http://dx.doi.org/10.7196/samj.2017.v107i7.11166
RESEARCH
An increase in rates of obstetric haemorrhage in a setting of high HIV seroprevalence
E ShabalalaI; H M SebitloaneII
IMB ChB. Department of Obstetrics and Gynaecology, Nelson R Mandela School of Medicine, College of Health Sciences, University of KwaZulu-Natal, Durban, South Africa
IIMB ChB, FCOG (SA), MMed, PhD. Department of Obstetrics and Gynaecology, Nelson R Mandela School of Medicine, College of Health Sciences, University of KwaZulu-Natal, Durban, South Africa
ABSTRACT
BACKGROUND. Obstetric haemorrhage (OH) is the leading cause of maternal mortality worldwide, although, indirectly, HIV is also a leading cause of maternal mortality in some settings with a high HIV seroprevalence.
OBJECTIVE. To determine the possible association between increasing rates of OH and HIV or its treatment.
METHODS. We conducted a retrospective chart review of women with OH at King Edward VIII Hospital, Durban, South Africa, over a 3-year period (2009 - 2011), during which the drug regimen for the prevention of mother-to-child transmission was evolving from single-dose nevirapine to antenatal zidovudine combined with intrapartum nevirapine (also referred to as dual therapy), and finally to a combination or highly active antiretroviral therapy (cART or HAART). Cases of OH (including abruptio placentae, placenta praevia, unspecified antepartum haemorrhage (APH), and postpartum haemorrhage (PPH)) were identified from maternity delivery records, and the relevant data extracted.
RESULTS. We analysed the records of 448 women diagnosed with OH. Even though the incidence of OH was low, the study found an increasing number of cases during the 3-year period. PPH - not APH - was associated with HIV seropositivity (odds ratio 1.84, 95% confidence interval 1.14 - 2.95). cART was not associated with an increased risk of haemorrhage.
CONCLUSION. HIV was associated with a high risk of PPH, and its possible association with HIV treatment needs further research.
Obstetric haemorrhage (OH) is the leading cause of maternal deaths worldwide, accounting for 25 - 30% of all such deaths, especially in low- to middle-income countries.[1] Non-pregnancy-related infections, where HIV is a major contributing factor, are the major cause of maternal deaths in sub-Saharan African countries, including South Africa (SA).[2] However, as this is an indirect cause, OH and hypertensive disorders of pregnancy (HDPs) are therefore the most common direct causes of maternal deaths.[2] In the initial SA triennial reports on maternal deaths, HDP was the overall leading cause of death and the leading cause of direct deaths. However, subsequently, HIV-related causes were predominantly in the lead. In SA, where 1 in 3 pregnant women are HIV infected,'31 the number of such deaths are now decreasing as a result of the successful use of combination antiretroviral treatment (cART), and consequently direct causes such as OH are beginning to emerge. An increase in OH, which mostly occurs at caesarean section, has already been shown in the last two triennial reports on maternal deaths.[3,4]
Pregnancy is a prothrombotic state, and in studies of adult patients, HIV has also been shown to be prothrombotic and may also be associated with deep vein thrombosis and thrombotic cerebrovascular accidents.[5] However, HIV is also associated with anaemia and thrombocyto-penia.'61 While there is a direct effect on haemostasis with the latter condition, anaemia has been cited as a risk factor for OH-associated morbidity.[6,7] HIV-infected women with advanced disease may have some form of chorioamnionitis, which may result in excessive postpartum haemorrhage (PPH). Subclinical chorioamnionitis may lead to higher rates of abruptio placentae, but also to poor postpar-tum contractility of the uterus.[8] It would be expected that with the widespread use of antiretroviral treatment, these effects would be reversed. Recently, an association between HIV infection and OH has been reported.[9,10] A closer look at the SA triennial reports show that, for the first time, there was an increased number of deaths from OH in the 2008 - 2010 report (and subsequent ones), 200 more than that recorded in the previous triennial reports of 2002 - 2004 and 2005 -2007.'41 This increase coincided with the introduction of cART among HIV-infected pregnant women, whereas previously, single-dose nevirapine (sdNVP) was administered. Against this background, we hypothesised that the increasing haemorrhage, as reported among maternal deaths, may be related to HIV or its treatment. We aimed to study this, using the available data.
Our study determined the prevalence of OH during the different phases of the changing drug regimen for the prevention of mother-to-child transmission (PMTCT).
Methods
We conducted a retrospective chart review of all recorded cases that had some form of OH at King Edward VIII Hospital, Durban, SA, from 1 January 2009 to 31 December 2011. About 500 - 600 neo-nates are delivered monthly at this large regional hospital. During this period, a number of changes were introduced with regard to antiretroviral drugs used in the PMTCT programme, including sdNVP (before 2008), and subsequently dual therapy (i.e. antenatal zidovudine from 28 weeks of gestation, intrapartum sdNVP for women with CD4 counts >200 cells/uL, and cART for those whose CD4 counts were <200 cells/uL). By the end of 2009, dual therapy was initiated from 14 weeks of pregnancy, and women were eligible for cART if their CD4 counts were <350 cells/uL. During the study period, cART consisted of stavudine, lamivudine and nevirapine.
We searched delivery records, theatre records and admissions to the high-care unit for the diagnosis of any form of OH. A case of OH was established if a record indicated a diagnosis of antepartum haemorrhage (APH) (unspecified), abruptio placentae, ruptured uterus, or retained placenta, bleeding after trauma to the lower genital tract, symptomatic placenta praevia, and PPH. We reviewed in-patient records of all identified and available cases and of those who had a blood transfusion (also in the case of a vaginal birth or any procedure for manual removal of the placenta) to determine if a diagnosis of APH or PPH was recorded. Data regarding patient demographics, obstetric complications and HIV status were entered into a Microsoft Excel spreadsheet and exported to SPSS version 21 (IBM Corp., USA) for analysis. Continuous variables were summarised using means, standard deviations (SDs) and odds ratios (ORs). Significant associations were identified using Fisher's exact test or the standard Pearson x2 test. Statistical significance was assessed at p<0.05.
The study was approved by the Biomedical Research Ethics Committee (ref. no. BE270/12) and King Edward VIII Hospital administration (ref. no. KE2/7/1/01/2013). As this was a retrospective study, informed consent was not obtained.
Results
According to the different registers used, 582 patients were diagnosed with some form of OH or with blood transfusion during the 3 years of the study. Fifty-four files of patients listed as OH cases could not be located. Fifty-seven, who were listed to have some form of OH, could also not be found, but this was not recorded in the patient file. Another 37 patients had been transfused for anaemia not associated with haemorrhage and were therefore excluded. Finally, data from 448 patient files were reviewed. During the study period, there were 19 953 deliveries in the hospital, with an incidence of 2.25% for OH. Thirty-four percent (n=153) of these were HIV-infected, 57.6% (n=258) HIV-uninfected, and in 8.3% (n=37) HIV was not recorded or HIV status was unknown.
The study found that as the PMTCT regimen evolved to include more drugs introduced at an earlier stage of gestation, the total number of OH cases increased with each year studied (Fig. 1). The annual incidence of OH was 1.63%, 2.49% and 2.61% for 2009, 2010 and 2011, respectively (p<0.0001). This increasing incidence coincided with the changing regimen of PMTCT in 2008 - 2009, from the administration of zidovudine at 28 weeks to a much earlier gestation at 14 weeks (Fig. 1). Parallel with this change, there was also an increase in the CD4 count threshold for the administration of cART, where women with CD4 counts of <350 cells/uL (not <200 cells/ uL) were now eligible for cART, i.e more women were now receiving cART.
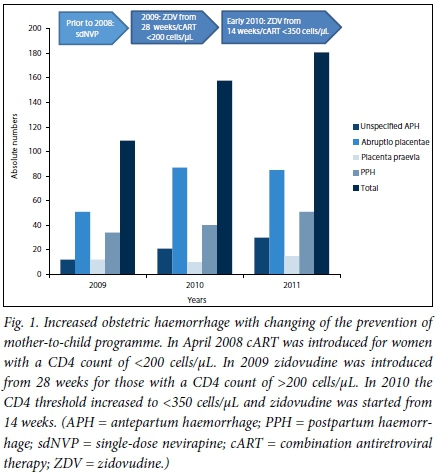
The most common condition among OH patients was abruptio placentae (n=271, 60.5%), followed by PPH (n=125, 27.9%). The latter followed unspecified APH in 69 women. In the remaining women, PPH was secondary to a retained placenta (n=14), uterine atony (n=27) and lower genital tract tears, including a ruptured uterus (n=15). The most common risk factor for abruptio placentae was HDP (including chronic hypertension and pre-eclampsia) (n=73, 26.9%), while a significant proportion had no risk factors (n=76, 28%). Unspecified APH accounted for 16.9% (n=76), possibly due to minor degrees of abruptio placentae. Placenta praevia accounted for 10.1% (n=45) of cases (Table 1).
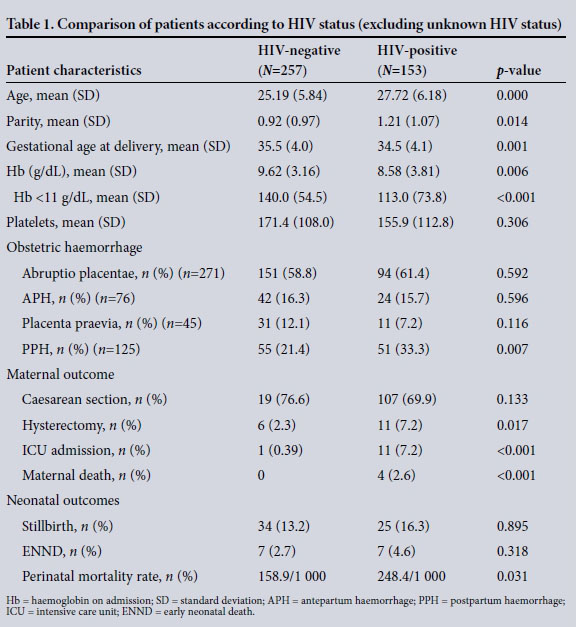
Of the 153 HIV-infected women, 24.8% (n=38) were on sdNVP, 69 (45.1%) received dual therapy, and 46 (30.1%) were on cART. The mean CD4 counts were 395 (range 50 - 1 000) cells/uL, 417 (177 - 720) cells/uL, and 273 (131 - 980) cells/uL for women receiving sdNVP, dual therapy and cART, respectively. Whereas HIV was associated with an increased risk of PPH (p=0.007), Table 2 shows no statistically significant association between OH and HIV treatment.
The proportion of women who developed abruptio placentae and unspecified APH was similar for HIV-infected (77.1%) and HIV-uninfected (74.8%) patients (p=0.596). In the HIV-infected group, there was no difference in the risk of abruptio placentae, unspecified APH and PPH between those who received highly active antiretroviral therapy (HAART) and those who received fewer than three drugs (i.e. sdNVP or dual therapy) (p=0.123, p=0.834 and p=0.901, respectively).
Maternal and perinatal outcomes
The mean (SD) age of the patients was 26.3 (6.325) years, with a parity of 1±1 (0 - 6) - HIV-infected women being slightly older (p=0.004) (Table 1). The gestational age at delivery was lower among HIV-infected women and those with unknown HIV status (p=0.005). Thirty-six percent of women delivered at <34 weeks, and 30% at or beyond 38 weeks of gestation, but these differences did not differ according to HIV status.
The mean haemoglobin (Hb) at baseline was 9.17 (2 - 16) g/dL, and was significantly lower among HIV-infected (8.6 g/dL) compared with HIV-uninfected (9.62 g/dL) patients (p=0.006). Similarly, there were more women with anaemia (Hb <11 g/dL) among HIV-infected than HIV-uninfected women (p=0.000). 0verall, 40.3% (n=178) of women required transfusion, receiving a mean of 2.8 U of packed cells, with more HIV-infected than HIV-uninfected women being transfused (p=0.045). The caesarean section rate was high (n=326, 72.6% ); however, it was similar in both groups (HIV-uninfected (76.6%) and HIV-infected (69.9%)) (p=0.133). PPH occurred at or after caesarean section in 40.8% (n=40) of cases. This difference of fewer caesarean sections among PPH cases compared with the entire group with OH was statistically significant (relative risk = 0.5, 95% confidence interval (CI) 0.39 - 0.64).
There were four maternal deaths among HIV-infected women, two of whom were receiving cART. They died in the intensive care unit (ICU) from hypovolaemic shock, subsequent disseminated intravascular coagulation and multi-organ failure - secondary to placenta praevia (n=1), PPH at caesarean section for fetal distress and cephalopelvic disproportion (with no other risk factors, n=2), and caesarean section for abruptio placentae (n=1). Furthermore, those who survived suffered severe morbidity, such as transfusion, renal failure, hysterectomy and ICU admission. The mortality risk, hysterectomy and ICU admission were all significantly associated with HIV-seropositive status (Table 1).
There were 365 (81.5%) live births, with 69 stillbirths (18.5%) and 14 early neonatal deaths. The perinatal mortality rate was much higher among HIV-infected patients (248/1 000 births) compared with HIV-uninfected patients (159/1 000) (p=0.031).
Discussion
We found that HIV increased the risk of PPH, but not of any form of APH. This is in keeping with the findings of Curtis et aZ.,[9] who showed an association between severe OH and positive HIV serostatus. However, Calvert and Ronsmans,[10] in a systematic review and meta-analysis, reported no increased odds of PPH from 13 studies, but twice the odds of APH. As mentioned above, the total number of haemorrhage cases per year increased significantly over the same period, during which more drugs were being added to the PMTCT regimen. We could, however, not demonstrate any significant association with the use of cART in any of the categories examined. It is also worth noting that the clinical implementation of the changing guidelines often lagged behind, and it is therefore difficult to make direct associations. In a secondary analysis of the data from the Saving Mothers Report of 2014, there was an increased risk of OH associated with both HIV infection and its treatment,[11] but this was not so in the current study.
The possible association between HIV and PPH is probably due to haemostatic factors or poor uterine contractility, as vascular factors at the uteroplacental interface would probably manifest with a higher occurrence of APH (e.g. abruptio placentae). Further studies are needed in this regard.
The prevalence of OH in our study was lower than that in other studies, which was unexpected, given the background reports. APH was 1.97% and PPH 0.63%. Both these figures are lower than those quoted in other studies (APH 3 - 5%™ and PPH 5 - 13%I13]), despite the local figures of increasing numbers of both conditions among maternal deaths, particularly PPH after caesarean sec-tion.[4] Lombaard and Pattinson[14] recorded a higher PPH incidence of 8 - 9% in their report of near misses among a population similar to that in the current study. We do not believe that this is an under-representation of the conditions examined, as we screened records from all the relevant sectors in the maternity sections. It is possible that there was under-reporting of PPH cases at vaginal delivery; however, this was probably clinically insignificant, as these cases would have been reported under blood transfusions. The caesarean section rate in the current cohort was high (>70%), which is understandable, as most causes of APH (placenta praevia, abruptio placentae with a live baby, and unclassified APH) require delivery by caesarean section. PPH after caesarean section occurred in only 40% of cases - the normal rate for this regional hospital. According to SA reports, there was an increase of 28% in the deaths from bleeding after caesarean section between 2011 and 2012 (27.5 - 35.3%). [2,4] It may be that in the majority of women who die of PPH the condition occurs during caesarean section. It may mean that the estimation of blood loss is inaccurate in these circumstances or, alternatively, that the resuscitative measures are insufficient or instituted too late. The poor estimation of blood loss may account for the lower prevalence of PPH after vaginal deliveries.
We found that HIV-infected women had significantly lower Hb values at the time of admission, with more of these women receiving blood transfusions. Bloch et al.,[15] in a large SA study, showed an increased risk of transfusions with blood products among HIV-infected pregnant women compared with HIV-uninfected women (3.7% v. 2.4%) (adjusted OR 1.52; 95% CI 1.14 - 2.03).
As can be expected, maternal morbidity from haemorrhage (e.g. in hysterectomy and maternal deaths) was higher in HIV-infected women, with a similar trend observed in perinatal outcomes. The latter showed higher rates of both stillbirths and early neonatal deaths in HIV-infected women, with an overall increased perinatal mortality rate among these women. The perinatal mortality rate was also increased among HIV-infected women (three times higher than the local figure (Ati MA, et al. - unpublished data) and probably related to abruptio placentae (>50% in the study population).
Study limitations
The study is limited by its retrospective nature, and some of the vital information, such as CD4 counts, was missing. The lack of control for CD4 counts, and the study being underpowered to show the differences according to the use of cART among HIV-infected women, are also noted as limitations. Both these are important areas to pursue in further research, as the ongoing monitoring of drug adverse effects is important.
Conclusion
The results of the current study add to the body of emerging reports that indicate a higher rate of OH in HIV-infected women. Even though there was originally an apparent increased risk of haemorrhage with the introduction of HIV treatment, this was not statistically ascertained in our study. An underlying mechanism could not be determined in our analysis; it therefore calls for further research to confirm the abovementioned findings and possible associated causes.
Recommendations
Reducing the number of deaths from OH is a priority worldwide, and the possible association between PPH and HIV makes it even more urgent. With the cART programme continuously evolving and the use of new drugs being introduced, it is important to continue monitoring adverse events.
Acknowledgements. None.
Author contributions. ES and HMS: concept generation and analysis/
writing of article; ES: data collection.
Funding. None.
Conflicts of interest. None.
References
1. Khan KSW. WHO analysis of causes ofmaternal death: A systematic review. Lancet 2006;367(9516):1066-1074. http://dx.doi.org/10.1016/S0140-6736(06)68397-9 [ Links ]
2. National Department of Health. National Committee on Confidential Enquiries into Maternal Deaths. Saving Mothers 2011 - 2013: Sixth Report on the Confidential Enquiries into Maternal Deaths in South Africa. Pretoria: NDoH, 2014. www.kznhedth.gov.za/mcwh/Maternal/Saving-Mothers-2011-2013-short-report.pdf (accessed 31 May 2017). [ Links ]
3. National Department of Health. 2012 National Antenatal Sentinel HIV & Herpes Simplex Type-2 Prevalence Survey in South Africa. Pretoria: NDoH, 2014. http://www.hst.org.za/publications/2012-national-antenatal-sentinel-hiv-herpes-simplex-type-2-prevalence-survey (accessed 9 May 2017). [ Links ]
4. National Department of Health. National Committee on Confidential Enquiries into Maternal Deaths. Saving Mothers 2008 - 2010: Fifth Report on the Confidential Enquiries into Maternal Deaths in South Africa. Pretoria: NDoH, 2012. www.kznhealth.gov.za/mcwh/Maternal/Saving-Mothers-2011-2013-short-report.pdf (accessed 31 May 2017). [ Links ]
5. Baker JV, Brummel-Ziedins K, Neuhaus J, et al. INSIGHT SMART study team HIV replication alters the composition of extrinsic pathway coagulation factors and increases thrombin generation. J Am Heart Assoc 2013;2(4):e000264. http://dx.doi.org/10.1161/JAHA.113.000264 [ Links ]
6. Parinitha S, Kulkarni M. Haematological changes in HIV infection with correlation to CD4 cell count. Australas Med J 2012;5(3):157-162. http://dx.doi.org/10.4066/AMJ.20121008 [ Links ]
7. Frass KA. Postpartum hemorrhage is related to the hemoglobin levels at labor: Observational study. Alexandria Med J 2015;51(4):333-337. http://dx.doi.org/10.1016/j.ajme.2014.12.002 [ Links ]
8. Nath CA, Ananth CV, Smulian JC, Shen-Schwarz S, Kaminsky L; New Jersey-Placental Abruption Study Investigators. Histologic evidence of inflammation and risk of placental abruption. Am J Obstet Gynecol 2007;197(3):e1-e6. http://dx.doi.org/10.1016/j.ajog.2007.06.012 [ Links ]
9. Curtis M, El Ayadi A, Mkumba G, et al. Association between severe obstetric hemorrhage and HIV status. Int J Gynaecol Obstet 2014;125(1):79-80. http://dx.doi.org/10.1016/j.ijgo.2013.10.010 [ Links ]
10. Calvert C, Ronsmans C. HIV and the risk of direct obstetric complications: A systematic review and meta-analysis. PLoS ONE 2013;8(10):e74848. http://dx.doi.org/10.1371/journal.pone.0074848.g001 [ Links ]
11. Sebitloane HM. Increasing maternal deaths due to obstetric hemorrhage in a setting of high HIV seroprevalence. Int J Gynaecol Obstet 2016;134(2):222-223. http://dx.doi.org/10.1016/j.ijgo.2016.02.012 [ Links ]
12. Bauserman M, Lokangaka A, Thorsten V, Tshefu A, Shivaprasad S, Esamai F. Risk factors for maternal death and trends in maternal mortality in low- and middle-income countries: A prospective longitudinal cohort analysis. Reprod Health 2015;12 (Suppl 2):S5. http://dx.doi.org/10.1186/1742-4755-12-S2-S5 [ Links ]
13. Calleja-Agius J, Custo R, Brincat MP, Calleja N. Placental abruption and placenta praevia. Eur Clin Obstet Gynaecol 2006;2(3):121-127. http://dx.doi.org/10.1007/s11296-006-0046-5 [ Links ]
14. Lombaard H, Pattinson R. Common errors and remedies in managing postpartum haemorrhage. Best Pract Res Clin Obstet Gynaecol 2009;23(3):317-326. http://dx.doi.org/10.1016/j.bpobgyn.2009.01.006 [ Links ]
15. Bloch EM, Crookes RL, Hull J, et al. The impact of human immunodeficiency virus infection on obstetric hemorrhage and blood transfusion in South Africa. Transfusion 2015;55(7):1675-1684. http://dx.doi.org/10.1111/trf.13040 [ Links ]
 Correspondence:
Correspondence:
H M Sebitloane
sebitloanem@ukzn.ac.za
Accepted 14 March 2017














