Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.107 no.7 Pretoria Jul. 2017
http://dx.doi.org/10.7196/samj.2017.v107i7.12309
RESEARCH
Incidence of chemotherapy-induced neutropenia in HIV-infected and uninfected patients with breast cancer receiving neoadjuvant chemotherapy
S NgidiI; N MagulaII; B SartoriusIII; P GovenderIV; T E MadibaV
IMB ChB, FC Rad One (SA); Department of Radiation Oncology, School of Clinical Medicine, College of Health Sciences, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
IIBSc, MB ChB, FCP (SA), MSc, PhD; Department of Internal Medicine, School of Clinical Medicine, College of Health Sciences, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
IIIPhD; Discipline of Public Health Medicine, School of Nursing and Public Health, College of Health Sciences, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
IVMB ChB, FC Rad Onc (SA), MMed; Department of Radiation Oncology, School of Clinical Medicine, College of Health Sciences, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
VMB ChB, MMed, LLM, PhD, FCS (SA), FASCRS;Department of Surgery, School of Clinical Medicine, College of Health Sciences, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
ABSTRACT
BACKGROUND. Chemotherapy-induced neutropenia (CIN) can result in poor tolerance of chemotherapy, leading to dose reductions, delays in therapy schedules, morbidity and mortality. Actively identifying predisposing risk factors before treatment is of paramount importance. We hypothesised that chemotherapy is associated with a greater increase in CIN and its complications in HIV-infected patients than in those who are not infected.
OBJECTIVE. To establish the incidence of CIN in HIV-infected and uninfected patients undergoing chemotherapy.
METHODS. A retrospective chart review and analysis was conducted in the oncology departments at Inkosi Albert Luthuli Central Hospital and Addington Hospital, Durban, South Africa. The study population consisted of 65 previously untreated women of all ages with stage II - IV breast cancer and known HIV status treated with neoadjuvant chemotherapy from January 2012 to December 2015.
RESULTS. HIV-infected patients formed 32.3% of the group, and 95.2% of them were on antiretroviral therapy. The mean age (standard deviation (SD)) of the cohort was 48.5 (13.2) years (40.6 (9.6) years for the HIV-infected group v. 52.0 (13.1) years for the uninfected group; p<0.001). Ninety-five neutropenia episodes were observed (rate 0.85 per 1 year of follow-up time). Following multivariate adjustment, patients with HIV infection were almost two times more likely to develop CIN (hazard ratio (HR) 1.76, 95% confidence interval (CI) 1.06 -2.92; p=0.029. A high baseline absolute neutrophil count (ANC) (HR 0.80, 95% CI 0.68 - 0.95; p=0.005) remained significantly associated with protection against CIN.
CONCLUSIONS. HIV-infected patients were younger than those who were not infected, and presented at a more locally advanced stage of disease. HIV infection was an independent predictor for CIN. HIV-infected patients had an almost two-fold increased risk of developing CIN and developed neutropenia at a much faster rate. A high baseline white cell count and ANC were protective against CIN.
Breast cancer is the most common cancer in females worldwide, representing one in four cancers in women.[1,2] It is the main cause of cancer-related death in women in less developed regions and the second most common cause in more developed countries.[3] Since the 2008 cancer incidence estimate,[4] the incidence has increased worldwide by >20% and mortality by 14%, with the most rapid increase occurring in many developing countries.[1] According to the South African National Cancer Registry in 2014,[5] breast cancer accounted for 20.64% of all cancers[5] and ranked second as the cause of cancer deaths in females.[6] The current statistics are unknown.
More than 40% of patients infected with HIV will develop cancer during their illness.[6] Internationally, there have been very few studies of breast cancer in relation to HIV infection. Most of the data generated in the developed world were based on relatively small study populations of up to 20 patients and did not show any relationship between breast cancer and HIV infection.[7-9] The few studies in South Africa (SA) had substantially larger study populations and failed to show an increased incidence of breast cancer among HIV-infected patients, but they did show that HIV-infected women were younger at the time of presentation.[9,10] The available evidence has also suggested that HIV-infected cancer patients in general have a worse prognosis than similarly staged non-infected patients with the same cancer, and that they are also likely to have more advanced disease at diagnosis.[11,12]
Chemotherapy-induced neutropenia (CIN) is one of the most serious haematological toxicities caused by chemotherapy.[13] Its prevalence in HIV-infected women with breast cancer is unknown. Most studies have reported on CIN and HIV infection in haematological malignancies, and few have looked at the relationship between CIN and HIV infection in solid tumours.[13] Because data on CIN in patients with breast cancer and HIV infection are scarce, little is known about its incidence and management. Furthermore, most publications on CIN in HIV-infected patients with cancer have emanated from countries in the developed world. Sub-Saharan Africa has the highest incidence of HIV infection in the world,[14] and KwaZulu-Natal (KZN) is the province with the highest rate of infection in SA.[15]
We hypothesised that HIV infection predisposes patients with breast cancer undergoing chemotherapy to CIN and its complications.
Objective
To compare HIV-infected and uninfected patients in terms of the incidence and severity of complications secondary to CIN.
Methods
A retrospective chart review of all patients who underwent chemotherapy for breast cancer over the 4-year period 1 January 2012 - 31 December 2015 at the oncology units at Inkosi Albert Luthuli Central Hospital and Addington Hospital in Durban, KZN, was conducted. These hospitals are tertiary referral centres for the coastal belt of KZN. Key data captured from the clinical notes are shown in Fig. 1. Primary prophylaxis was defined as the use of granulocyte colony-stimulating factor (G-CSF) up front from the first cycle of chemotherapy until the end, and secondary prophylaxis as use of G-CSF in response to a neutropenic complication.

Chemotherapy regimens offered in the oncology units are shown in Fig. 2. These regimens are considered to pose an intermediate (10 - 20%) risk of febrile neutropenia.[16,17] Neutropenia was defined according to the Common Toxicity Criteria of the National Cancer Institute as a disorder characterised by an absolute neutrophil count (ANC) of <2 x 109/L.[18,19] Neutropenia is categorised into four grades, depending on the ANC.
Patients
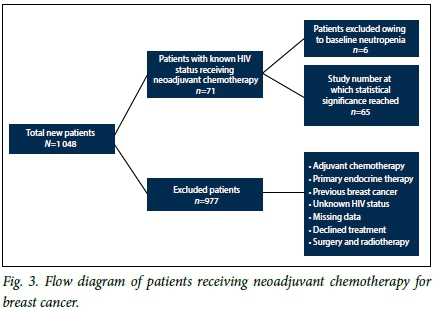
The study population consisted of previously untreated newly diagnosed patients of all ages with documented HIV status and histologically confirmed breast cancer who received neoadjuvant chemotherapy. The cohort consisted of women only, as men did not fulfil the eligibility criteria. The total number of new female patients diagnosed with breast cancer in the study period was 1 048, with the average number of new cases per year being 262. The cohort had stage II - IV breast cancer. Stage IV patients had oligometastases and received radical treatment. To minimise confounding factors, we excluded patients with baseline pre-existing neutropenia, those with a history of previous chemotherapy or a history of breast cancer or other prior or existing cancers, those with active infection, those receiving concurrent treatment with medication that predisposes to neutropenia, such as long-term steroids, quinidine and hydralazine, those who had stopped neoadjuvant chemotherapy prematurely for any reason other than neutropenia, and those for whom critical data in charts were missing (Fig. 3).
Statistical analysis
Data were analysed using Stata version 13.0 (StataCorp, USA). Continuous variables were summarised using means, standard deviations (SDs) and ranges. If data were skewed, medians and ranges were presented. Significant associations in contingency tables (cross-tabulations) were assessed using Pearson's x2 test. If an expected cell count in the cross-tabulation contained <5 observations, Fisher's exact test was used instead. Time-to-event (survival) analysis was employed to estimate the overall incidence of neutropenia and associated 95% confidence intervals (CIs). We constructed Kaplan-Meier failure curves to assess differences in failure by key covariates. 'Failure' was defined as failure of the patient to maintain a normal neutrophil level, i.e. development of neutropenia. We further extended our analysis to employ bivariate and multivariate survival modelling (Cox proportional hazards approach) to identify covariates of incident neutropenia. An adjusted p-value of <0.05 was considered statistically significant. Only factors that were statistically significant on the unadjusted logistic regression analysis were entered into the adjusted logistic regression model.
Ethics approval
The study was approved by the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (ref. no. BE: 094/14) and the Provincial Health Research Committee, KZN Department of Health (same reference number used).
Results
Patient characteristics
Of a total of 1 048 patients with breast cancer, 71 fulfilled the eligibility criteria. Subjects with pre-existing neutropenia at baseline (n=6) were excluded from the final analysis, yielding a final sample size of 65 (Fig. 3). Baseline characteristics of the cohort are shown in Table 1. Twenty-one patients (32.3%) were HIV-infected, of whom 20 (95.2%) were on antiretroviral therapy (ART). The mean (SD) CD4 count was 435 (248) cells/uL (range 80 - 945). HIV viral loads were documented in only four patients. The mean age was 48 (13.2) years (range 24 - 80). The HIV-infected group was younger than the uninfected group (mean age 40 (9.6) years (range 24 - 58) v. 52 (13) years (range 30 - 80); p<0.001).
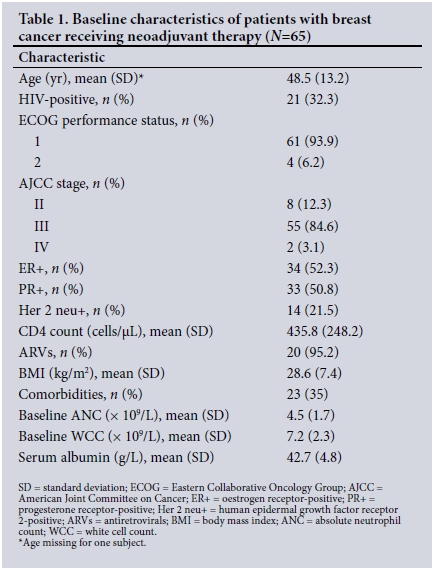
Sixty-one patients (93.8%) had good performance status (PS1). Twenty-five (38.5%) had comorbidities (p=0.072), including hypertension (n=17, 68.0%), diabetes mellitus (n=11, 44.0%), osteoarthritis (n=6, 24.0%), dyslipidaemia (n=3, 12.0%), asthma (n=3, 12.0%) and ischaemic heart disease (n=2, 8.0%). Renal failure, hypothyroidism, cardiomyopathy, valvular heart disease and dermatomyositis constituted 3% of all comorbidities. Some patients had more than one comorbidity.
The mean (SD) baseline ANC was 4.9 (1.8) x 109/L (range 2.1 -8.4) and the mean baseline white cell count (WCC) 7.9 (2.2) x 109/L (range 3.3 - 14.9). A total of 33 patients (50.8%) experienced CIN; 14 (66.7%) were HIV-infected and 19 (43.2%) were uninfected. The mean CD4 count in the neutropenic HIV-infected patients was 378 cells/uL (range 80 - 708), compared with 509 cells/uL (range 245 - 945) in the non-neutropenic group. There were 95 episodes of neutropenia, ranging from one to six episodes per patient. This translated into 0.85 neutropenia episodes per 1-year follow-up time (95% CI 0.69 - 1.04).
Table 2 compares baseline characteristics of the HIV-infected and non-infected patients. Compared with the uninfected group, patients with HIV infection had a lower body mass index (BMI) (p=0.24) and serum albumin level, but the latter difference was not significant (p=0.168).
Fifty-five patients (84.6%) presented with stage III disease; 35 HIV-uninfected patients (79.5%) and 20 HIV-infected patients (95.2%) had stage III disease, suggesting that locally advanced disease was the predominant stage of presentation. These differences were not statistically significant (p=0.258). There was a significantly higher rate of endocrine unresponsiveness in the HIV-infected patients compared with those who were not infected.
Four chemotherapy regimens were used (Fig. 2). FEC was received by a total of 25 patients (38.5%), of whom 6 were HIV-infected. FEC-D was received by 32 patients (49.2%), with an equal distribution of HIV-infected and uninfected patients. TC was received by 6 patients (9.2%), of whom only 1 was HIV-infected. AC-D was received by 2 uninfected patients (3.1%).
Neutropenia
The ratio of neutropenic episodes in HIV-infected v. uninfected patients was 3:1. The baseline ANC was significantly lower in HIV-infected than in uninfected patients, and the mean WCC was also lower. This trend was maintained throughout chemotherapy (Fig. 4). Grades of neutropenia experienced by the HIV-infected v. the uninfected patients are shown in Table 3.
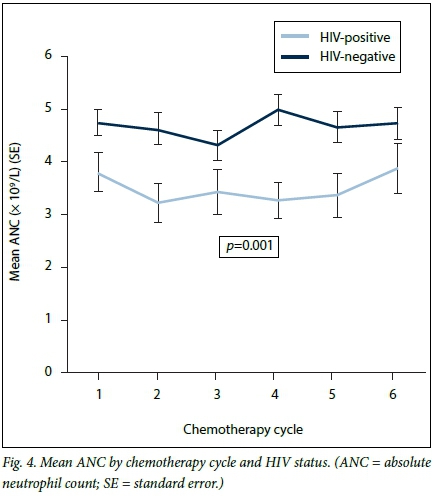
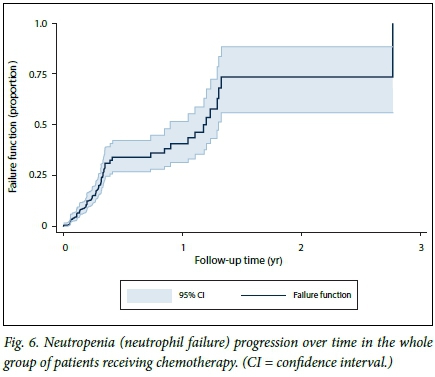
In the HIV-infected group, the lowest ANC from baseline was seen after the first chemotherapy infusion, namely day 1 of cycle two. The lowest ANC in the HIV-uninfected group was seen after the second chemotherapy infusion, namely day 1 of cycle three (Fig. 4). The mean ANCs recorded at day 1 of chemotherapy as well as in between chemotherapy cycles were lower in the HIV-infected patients than in those who were not infected (2 - 3 x 109/L v. 2 - 5 x 109/L; p<0.001) (Figs 4 and 5). Fig. 5 shows patients who experienced neutropenia and its complications in between cycles, the mean ANCs of those who were HIV-infected being lower than in those who were not (p<0.001). Mean levels declined in both groups, however, converging to a single level towards the end of chemotherapy. At 3.5 months (half way through chemotherapy), 25.0% of the overall cohort had developed neutropenia, while at 1 year 50.0% (median failure time) of the overall cohort had experienced neutropenia (Fig. 6).
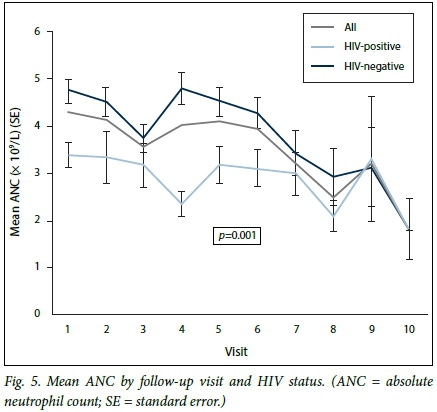
The proportions of neutropenic patients in each chemotherapy regimen were as follows. FEC-D: 15 patients (46.9%) experienced neutropenia, 10 (66.7%) HIV-infected v. 5 (33.3%) uninfected; FEC: 15 patients (60.0%) experienced neutropenia, 4 (26.7%) HIV-infected v. 11 (73.3%) uninfected; and TC: 3 patients (50.0%) experienced neutropenia, all HIV-uninfected. Neither of the 2 patients treated with AC-D experienced neutropenia.
Factors associated with neutropenia
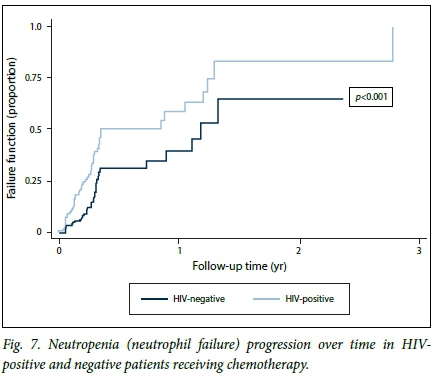
At regression analysis, HIV status was a significant predictor of neutropenia (unadjusted hazard ratio (HR) 2.1, 95% CI 1.39 - 3.18; p<0.001). After adjustment for age, HIV remained a significant predictor of neutropenia (HR 1.76, 95% CI 1.06 - 2.92; p=0.029). A higher baseline ANC remained a significant factor protecting against the development of neutropenia (HR 0.80, 95% CI 0.68 -0.95; p=0.005) (Table 4). Neutropenic failure with time was greater in the HIV-infected group than in the uninfected group. HIV-infected patients developed neutropenia at a much faster rate, i.e. 50.0% had already developed neutropenia at 3.5 months (half way through the chemotherapy schedule) compared with 30.0% of uninfected patients at the same point. This difference was statistically significant (p<0.001) (Fig. 7).
Response to neutropenia, clinical manifestations and sequelae
Interventions in patients who developed neutropenic complications included dose reduction, chemotherapy delays and G-CSF support.[12,20] Of the 33 patients who experienced frequent neutropenic episodes, 2 (6.1%) had 25% dose reductions of their chemotherapy regimen. Chemotherapy delays ranging from 7 days to 5 weeks occurred in the neutropenic group. The median delay was 3 weeks (range 1 - 5) in the HIV-infected patients compared with 1 week (range 1 - 2) in those who were not infected. Secondary G-CSF support was given to 9 patients (28.1%), with 3 receiving secondary prophylactic G-CSF support from cycle two (filgrastim 300 ug/d subcutaneously for 3 days). All but one event occurred in the HIV-infected group (71.4% v. 5.3%).
There was no clear documentation of the presence or absence of infections and fever at the time of detection of neutropenia. Patients were recorded as having had lower respiratory infections (n=4), diarrhoea (n=2), herpes simplex infection (n=1) and herpes zoster infection (n=1).
One episode of febrile neutropenia in one patient occurred after the first cycle of chemotherapy. The patient had HIV infection, with a baseline CD4 count of 175 cells/uL and a viral load of 822 940 copies/L, and was not on ART. Her septic screen was negative and she was given intravenous antibiotics and prophylactic G-CSF support (filgrastim 300 ug/d subcutaneously for 3 days), and was in hospital for 9 days. There were no further neutropenic episodes in subsequent cycles, as she received secondary prophylaxis.
One patient with neutropenia did not complete her chemotherapy schedule, which was aborted after two cycles owing to inability to achieve normal ANC levels after the first dose of chemotherapy. She had six recorded episodes of grade 2 - 3 neutropenia over a period of 14 weeks during her chemotherapy schedule. The complication experienced was a respiratory infection, which was managed on an outpatient basis with oral antibiotics. She was 50 years old, with HIV infection and stage III breast cancer. She had a CD4 count of 477 cells/uL and a viral load of 23 296 copies/L, but was not on ART. She was commenced on tamoxifen 20 mg/d, and the tumour was deemed resectable 7 months later. None of the study patients died.
Discussion
Neutropenia can result in poor tolerance to chemotherapy, leading to dose reductions, delays in chemotherapy administration, morbidity and mortality, and can ultimately influence treatment outcomes.[13,18] Active identification of predisposing risk factors prior to commencing chemotherapy is essential. In this study, HIV infection was shown to be an independent predictor of CIN. Decreased immunity due to active infection, myelosuppressive drugs and comorbidities have been reported as risk factors for developing CIN,[18-22] but HIV infection as an independent predictor of CIN in patients with solid tumours has not been reported. Neutropenia is a significant independent risk factor for bacteraemia in HIV-positive patients. It has been reported to occur in 35 - 75% of patients with AIDS.[23] CIN also occurred at a much faster rate in patients with HIV infection in our cohort. We postulate that this is because HIV-negative patients have an immune advantage over infected patients. HIV infection causes neutrophil dysfunction in a number of ways, including impaired chemotaxis and phagocytosis, an accelerated number of apoptotic neutrophils, expression of cellular adhesion molecules at the surface of the neutrophils, and production of toxic oxygen species.[23] Neutropenia and neutrophil dysfunction associated with HIV infection are partly mediated by abnormal regulation of cytokines.[23] In response to neutropenia, cytokines are released to increase the production of neutrophils. In the presence of HIV infection this is impaired somewhat, resulting in the neutropenic patient recovering much more slowly, with increased episodes and a more severe degree of neutropenia than in an HIV-uninfected neutropenic patient. HIV-infected patients may have other compounding morbidities such as opportunistic infections and manifestations of AIDS, and some may already have a baseline neutropenia due to their HIV infection before they start chemotherapy.[23] Furthermore, malignancy in itself can cause myelosuppression.[23,24] These factors are thought to contribute to HIV-infected patients' higher risk of developing neutropenia.
We also showed that a higher baseline ANC and WCC were associated with 18% protection against CIN. Baseline ANC was also established as a means of predicting the development of CIN in a study by Jenkins and Freeman.[25] Retrospective studies have demonstrated the predictive value of pre-treatment WCCs[24-27] and first-cycle nadir WCCs for predicting neutropenic complications in later cycles.[26] The baseline ANC is indicative of bone marrow reserve. Nadir ANCs and WCCs were much lower in the HIV-infected patients in our study compared with the HIV-uninfected patients, and this trend seemed to be maintained throughout the chemotherapy cycles. Mean ANC levels in the HIV-infected patients who experienced neutropenia were initially much lower than those in uninfected patients, but in the last few visits towards the end of chemotherapy the mean ANC levels of the two groups converged as the mean ANC of the HIV-uninfected group continued to decline at a relatively higher rate. It is not known why this occurred, but it is postulated that the initial advantage of a higher mean ANC in the uninfected patients was lost towards the end of treatment as their bone marrow haematopoietic reserve decreased.
Sequential chemotherapy appears to increase the likelihood of an HIV-infected patient experiencing neutropenia. More HIV-infected patients in our FEC-D group than on other regimens developed neutropenia. The group receiving FEC alone had more HIV-uninfected patients who experienced neutropenia. The reason for this is unknown.
ART did not prevent patients with HIV infection from experiencing neutropenia, but it did protect them from developing higher grades of neutropenia and more severe complications. The two neutropenic patients who were not on ART had CIN throughout chemotherapy, with higher grades resulting in severe complications and the need for G-CSF support and even to stop chemotherapy.
This study has shown that HIV-infected patients with breast cancer present at a significantly younger age than their uninfected counterparts. Cancers do occur at an earlier age in both women and men with HIV infection than in non-infected individuals.[28] SA studies on breast cancer have also found that HIV-infected patients presented at a much younger age and at a more advanced stage than those who were uninfected.[9,10] In our study, HIV-infected patients tended to present with more locally advanced disease, although this was not statistically significant. There are studies that support our finding of HIV-infected cancer patients being likely to have more advanced disease at diagnosis than similarly staged non-infected patients, and to have a poorer prognosis.[7,11,12,28] This could be due to the direct and indirect effects of the HIV virus on cancer biology. An indirect consequence of HIV infection is long-term immune suppression and alterations.[28] The more direct effects of the HIV virus include HIV tat protein transactivation of cellular genes or proto-oncogenes. Numerous HIV genes have been reported to inhibit tumour suppressor genes such as p53.[28] Impairment of the immune system and the ability of cancer cells to evade the immune system has been established as a hallmark in cancer biology.[29] Since the introduction of ART, reduction in other causes of death and better supportive care, patients with HIV have been living longer, and it is unknown whether the incidence of breast cancer will increase.[28] Even though ART has had a massive impact on cancer in the HIV setting, infected patients are still at higher cancer risk than the general population. An explanation of the increased risk is that, despite improved immune function on ART, cancer immune surveillance is still inadequate in patients with HIV.[30] Furthermore, HIV-infected women are less likely than those who are uninfected to undergo routine screening mammography, which may ultimately play a role in later diagnosis and more advanced presentation of breast cancer.[11,31] A high proportion of HIV-infected patients are young, and routine screening methods and education have largely targeted older women. Whether HIV infection alters the presentation or outcome of breast cancer is unknown,[9] as there have not yet been any large prospective cohort studies.[8,11]
Reduction of a patient's chemotherapy dose in response to neutropenia may lead to improvement of the neutropenia. However, like many other drugs, chemotherapeutic drugs have a dose-response relationship.[13,22] It has been demonstrated that decreasing the dose in patients with breast cancer may decrease efficacy.[22] Dose reduction is therefore not an ideal option, and growth factor support should be considered as an alternative.[21,22] Evidence suggests that prevention of neutropenia with G-CSF is more beneficial than treatment of established neutropenia.[21] Most neutropenic episodes occur in the early cycles of chemotherapy, and its early development has an influence on tolerance of subsequent cycles. Early use of G-CSF affects the risk of neutropenia in later cycles, perhaps owing to the priming effect on myeloid precursors.[13,32]
An unexpected finding was that increasing age was marginally associated with a protective effect following multivariate adjustment. Age in this instance may be a confounding factor, as HIV-infected patients tend to be relatively young and CIN was more common in HIV-infected patients. Other studies do not support this observation, and have found older age to be a risk factor for CIN.[24]
Eastern Cooperative Oncology Group (ECOG) performance status, TNM stage, BMI, serum albumin level and the presence of comorbidities had no influence on CIN in this series. This is not in keeping with the findings of other studies in which these factors have been shown to influence CIN, resulting in their being incorporated into oncology guidelines.[16,17,21]
Recommendations
We are in agreement with Schouten[22] that primary G-CSF prophylaxis should be considered in patients with HIV infection who receive chemotherapy for breast cancer. In endocrine-responsive HIV-infected patients, who have a >20% risk of developing neutropenia, we suggest primary endocrine therapy as an alternative to chemotherapy in the neoadjuvant setting. Endocrine therapy has been used successfully in this setting[33-36] owing to its favourable side-effect profile.[37] Routine HIV testing of all patients diagnosed with cancer should become a standard. Those who are infected should have their CD4 cell count and HIV viral load measured. Evidence suggests that it is beneficial to start these patients on ART prior to any oncological treatment.[38,39] We suggest that criteria for chemotherapy parameters (ANC and WCC) in HIV-infected patients should be modified to account for their lower ANC trends from baseline to completion, as this seems to be the norm for this group.
Study limitations
This was a retrospective study, and it relied completely on previously recorded data. A number of factors negatively affected the study, including incomplete documentation, test results and recording of HIV status. A larger prospective study is likely to strengthen the findings of this study.
Conclusions
This study has established HIV infection as an independent predictor of CIN, with HIV-infected patients being two times more likely to experience CIN and having a faster development rate than those who are uninfected. Higher baseline WCCs and ANCs are protective against developing CIN, and vice versa. HIV-infected patients should be regarded as at high risk of developing CIN, and primary prophylaxis should therefore be considered.
Acknowledgements. None.
Author contributions. Conception and design: SN; collection of data and assembly: SN; data analysis and interpretation: SN, BS; manuscript writing and editing: all authors; final approval of manuscript: all authors; critical revision for intellectual content: TEM.
Funding. This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Conflicts of interest. None.
References
1. Global cancer burden rises to 14.1 million new cases in 2012: Marked increase in breast cancers must be addressed. Cancer IAFRO, 2013. http://www.iarc.fr/en/media-center/pr/2013/pdfs/pr233_E.pdf (accessed 19 February 2016). [ Links ]
2. Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer, 2013. http://globocan.iarc.fr (accessed 23 October 2016). [ Links ]
3. Vorobiof DA, Sitas F, Vorobiof G. Breast cancer incidence in South Africa. J Clin Oncol 2001;19(Suppl 18):125-127. [ Links ]
4. Ferlay J, Shin H-R, Bray F, Forman D, Mathers C, Parkin DM. Estimates ofworldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127(12):2893-2917. https://doi.org/10.1002/ijc.25516 [ Links ]
5. Sitas F. Histologically diagnosed cancers in South Africa, 1988. S Afr Med J 1994;84(6):344-348. [ Links ]
6. Hurley J, Franco S, Gomez-Fernandez C, et al. Breast cancer and human immunodeficiency virus: A report of 20 cases. Clin Breast Cancer 2001;2(3):215-220. https://doi.org/10.1016/S1526-8209(11)70416-5 [ Links ]
7. Patel P, Hanson DL, Sullivan PS, et al. Incidence oftypes of cancer among HIV-infected persons compared with the general population in the United States, 1992 - 2003. Ann Intern Med 2008;148(10):728-736. http://doi.org/10.7326/0003-4819-148-10-200805200-00005 [ Links ]
8. Palan M, Shousha S, Stebbing J. Breast cancer in the setting of HIV. Pathol Res Int 2011;2011:1-4. http://dx.doi.org/10.4061/2011/925712 [ Links ]
9. Cubasch H, JofFe M, Hanisch R, et al. Breast cancer characteristics and HIV among 1,092 women in Soweto, South Africa. Breast Cancer Res Treat 2013;140(1):177-186. https://doi.org/10.1007/s10549-013-2606-y [ Links ]
10. Langenhoven L, Barnardt P, Neugut AI, Jacobson JS. Phenotype and treatment of breast cancer in HIV- positive and-negative women in Cape Town, South Africa. J Glob Oncol 2016;2(5):284-291. https://doi.org/10.1200/JGO.2015.002451 [ Links ]
11. De Andrade ACV, Luz PM, Veloso VG, et al. Breast cancer in a cohort of human immunodeficiency virus (HIV)-infected women from Rio de Janeiro, Brazil: A case series report and an incidence rate estimate. Braz J Infect Dis 2011;15(4). http://dx.doi.org/10.1590/S1413-86702011000400016 [ Links ]
12. Voutsadakis IA, Silverman LR. Breast cancer in HIV-positive women: A report of four cases and review of literature. Cancer Invest 2002;20(4):452-457. http://dx.doi.org/10.1081/CNV-120002144 [ Links ]
13. Crawford J, Dale DC, Lyman GH. Chemotherapy-induced neutropenia: Risks, consequences and new directions for its management. Cancer 2004;100(2):228-237. https://doi.org/10.1002/cncr.20218 [ Links ]
14. UNAIDS. Reports on the global epidemic 2008. http://www.unaids.org/site/default/file/media_asset/jc1510_2008globalreport_en_0.pdf (accessed 29 May 2016). [ Links ]
15. Shisana O, Labadarios D, Simbayi L, et al. South African National HIV Prevalence, Incidence and Behaviour Survey, 2012. Cape Town: HSRC Press, 2014. [ Links ]
16. Crawford J, Althaus B, Armitage JO, et al. Myeloid growth factors. Clinical practice guidelines in oncology. J Natl Compr Canc Netw 2007;5(2):188-202. http://dx.doi.org/10.6004/jnccn.2007.0019. [ Links ]
17. Aapro MS, Cameron DA, Pettengell R, et al. EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphomas and solid tumours. Eur J Cancer 2006;42(15):2433-2453. https://doi.org/10.1016/jejca.2006.05.002 [ Links ]
18. Crawford J, Dale DC, Lyman GH. Chemotherapy-induced neutropenia: Risks, consequences, and new directions for its management. Cancer 2004;100(2):228-237. https://doi.org/10.1002/cncr.11882 [ Links ]
19. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the radiation therapy oncology group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995;31(5):1341-1346. https://doi.org/10.1016/0360-3016(95)00060-C [ Links ]
20. Chen K, Zhang X, Deng H, et al Clinical predictive models for chemotherapy-induced neutropenia in breast cancer patient: A validation study. PLoS One 9(6):e96413. https://doi.org/10.1371/journal.pone.0096413 [ Links ]
21. Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 update of recommendations for the use of white blood cell growth factors: An evidence-based clinical practice guideline. J Clin Oncol 2006;24(19):3187-3205. https://doi.org/10.1200/JCO.2006.06.4451 [ Links ]
22. Schouten H. Neutropenia management. Ann Oncol 2006;17(Suppl 10):x85-x89. https://doi.org/10.1093/annonc/mdl243 [ Links ]
23. Kuritzkes DR. Neutropenia, neutrophil dysfunction, and bacterial infection in patients with human immunodeficiency virus disease: The role of granulocyte colony-stimulating factor. Clin Infect Dis 2000;30(2):256-260. https://doi.org/10.1086/313642 [ Links ]
24. Lyman GH, Lyman CH, Agboola O, for the ANC Study Group. Risk models for predicting chemotherapy- induced neutropenia. Oncologist 2005;10(6):427-437. https://doi.org/10.1634/theoncologist.10-6-427 [ Links ]
25. Jenkins P, Freeman S. Pre-treatment haematological laboratory values predict for excessive myelosuppression in patients receiving adjuvant FEC chemotherapy for breast cancer. Ann Oncol 2009;20(1):34-40. https://doi.org/10.1093/annonc/mdn560 [ Links ]
26. Silber JH, Fridman M, DiPaola RS, Erder MH, Pauly MV, Fox KR. First-cycle blood counts and subsequent neutropenia, dose reduction, or delay in early-stage breast cancer therapy. J Clin Oncol 1998;16(7):2392-2400. [ Links ]
27. López-Pousa A, Rifà J, Casas de Tejerina A, et al Risk assessment model for first-cycle chemotherapy- induced neutropenia in patients with solid tumours. Eur J Cancer Care 2010;19(5):648-655. https://doi.org/10.1111/j.1365-2354.2009.01121.x [ Links ]
28. Mitsuyasu R. Non-AIDS-defining malignancies in HIV. Top HIV Med 2008;16(4):117-121. [ Links ]
29. Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell 2011;144(5):646-674. https://doi.org/10.1016/j.cell.2011.02.013 [ Links ]
30. Bedimo R, Chen RY, Accortt NA, et al Trends in AIDS-defining and non-AIDS-defining malignancies among HIV-infected patients: 1989 - 2002. Clin Infect Dis 2004;39(9):1380-1384. https://doi.org/10.1086/424883 [ Links ]
31. Krishnan A, Levine AM. Malignancies in women with HIV infection. Womens Health 2008;4(4):357-368. [ Links ]
32. Crawford J. Pegfilgrastim for the prevention of chemotherapy-induced neutropenic complications, with dosing once per chemotherapy cycle. Todays Ther Trends 2002;S20(4):393-418. [ Links ]
33. Cataliotti L, Buzdar AU, Noguchi S, et al Comparison of anastrozole versus tamoxifen as preoperative therapy in postmenopausal women with hormone receptor-positive breast cancer. Cancer 2006;106(10):2095-2103. https://doi.org/10.1002/cncr.21872 [ Links ]
34. Masuda N, Sagara Y, Kinoshita T, et al. Neoadjuvant anastrozole versus tamoxifen in patients receiving goserelin for premenopausal breast cancer (STAGE): A double-blind, randomised phase 3 trial. Lancet Oncol 2012;13(4):345-352. https://doi.org/10.1016/51470-2045(11)70373-4 [ Links ]
35. Smith IE, Dowsett M, Ebbs SR, et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: The Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol 2005;23(22):5108-5116. https://doi.org/10.1200/JCO.2005.04.005 [ Links ]
36. Eiermann W, Paepke S, Appfelstaedt J, et al. Preoperative treatment of postmenopausal breast cancer patients with letrozole: A randomized double-blind multicenter study. Ann Oncol 2001;12(11):1527-1532. https://doi.org/10.1023/A:1013128213451 [ Links ]
37. Charehbili A, Fontein D, Kroep J, et al. Neoadjuvant hormonal therapy for endocrine sensitive breast cancer: A systematic review. Cancer Treat Rev 2014;40(1):86-92. https://doi.org/10.1016/j.ctrv.2013.06.001 [ Links ]
38. Kaplan JE, Benson C, Holmes KK, et al. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents. MMWR Recomm Rep 2009;58(RR-4):1-207. [ Links ]
39. Torres HA, Mulanovich V. Management of HIV infection in patients with cancer receiving chemotherapy. Clin Infect Dis 2014;59(1):106-114. https://doi.org/http://doi.org/10.1093/cid/ciu174 [ Links ]
 Correspondence:
Correspondence:
S Ngidi
drsngidi@gmail.com
Accepted 6 March 2017.














