Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.107 n.6 Pretoria Jun. 2017
http://dx.doi.org/10.7196/samj.2017.v107i6.12099
IN PRACTICE
CLINICAL UPDATE
Establishing an academic biobank in a resource-challenged environment
C C SooI; F MukomanaII; S HazelhurstIII, IV; M RamsayIII, V
IMSc; Sydney Brenner Institute for Molecular Bioscience, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIBSc (Hons); Sydney Brenner Institute for Molecular Bioscience, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIIPhD; Sydney Brenner Institute for Molecular Bioscience, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IVPhD; School of Electrical and Information Engineering, Faculty of Engineering and the Built Environment, University of the Witwatersrand, Johannesburg, South Africa
VPhD; Division of Human Genetics, School of Pathology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
Past practices of informal sample collections and spreadsheets for data and sample management fall short of best-practice models for biobanking, and are neither cost effective nor efficient to adequately serve the needs of large research studies. The biobank of the Sydney Brenner Institute for Molecular Bioscience serves as a bioresource for institutional, national and international research collaborations. It provides high-quality human biospecimens from African populations, secure data and sample curation and storage, as well as monitored sample handling and management processes, to promote both non-communicable and infectious-disease research. Best-practice guidelines have been adapted to align with a low-resource setting and have been instrumental in the development of a quality-management system, including standard operating procedures and a quality-control regimen. Here, we provide a summary of 10 important considerations for initiating and establishing an academic research biobank in a low-resource setting. These include addressing ethical, legal, technical, accreditation and/or certification concerns and financial sustainability.
The need to establish and develop formal biobanks in South Africa (SA), especially for human biological samples, is increasing.[1] Internationally funded initiatives such as Human Heredity and Health in Africa (H3Africa), and various investments into HIV, tuberculosis (TB) and malaria research, are creating awareness of the value of high-quality human biospecimen availability for health and disease research.[1] As such, academic institutions find themselves at the forefront of a drive to invest in the development of secure, affordable and convenient storage facilities for human samples. This fast-evolving scientific field brings with it many social and ethical issues that require careful consideration.[2,3] The University of the Witwatersrand, Johannesburg (Wits), has taken the lead in promoting the establishment of high-quality biobanks and biobanking practices in the region, and in addressing the legal and ethical implications for biobanking in SA.[2]
The Sydney Brenner Institute for Molecular Bioscience (SBIMB) is a cross-disciplinary research institute at Wits. The institute offers postgraduate students and collaborating researchers the resources to perform high-quality and high-impact research with a focus on critical questions related to the health of African populations. The SBIMB has a growing platform of expertise in the fields of human genetics and genomics, population genetics, bioinformatics, evolutionary biology and biomedical informatics, with an extensive network of collaborators globally. Our objective, to develop capacity for genomic research in Africa, is in line with that of the H3Africa consortium, and our involvement in studies of African and SA population genetics and clinical studies in ophthalmology, rheumatology, cancers, cardiometabolic diseases and obesity is all focused on African cohorts. This article documents the challenges experienced in the establishment of the SBIMB biobank and specific considerations for biobanking in SA, as well as providing a summary of 10 critical issues to address before embarking on a biobanking venture in a low-resource setting.
Governance
A well-established system of governance is essential for the development of good policies, and for monitoring their implementation. Managing ethical, legal and social implications is critical for any biobank, and the attendant regulations will necessarily and desirably be burdensome. The SA legal framework currently makes no provision for the regulation or governance of biobanking.[2-4] The amendments made in 2012 to the National Health Act No. 61 of 2003 apply to tissue and stem-cell banking for clinical purposes, but not to biobanking in general.[5] They leave the regulation of biobanks in the hands of ethics regulatory bodies, rather than within a legal framework, and the regulations regarding the storage, use and re-use of DNA are vague.[2,4,6)
Recommendations for regulations specific to biobanking in SA and across national African borders have been made, and an important issue that was raised was genomic sovereignty.[4,6] Developing genomic capacity in Africa, rather than exporting DNA samples for storage and analysis to higher-income countries, is essential.[6] The inherent genomic diversity of African populations is important in order to better understand disease aetiology. To prevent exploitation, special attention should be given to drafting material transfer agreements (MTAs) that specifically outline benefits to both parties, and disallow misuse or further use of samples for purposes other than those of the original agreement, unless agreed to in writing.[2,6] Through the Wits Biobanks Ethics Committee (BEC), an MTA document was drafted and approved for use for Wits research.[4,7] This document emphasises the importance of informed consent, benefit sharing and ownership of biospecimens through transfer across national boundaries.[4] It is available on the Wits website, and is a valuable guideline when drafting MTAs for the transfer of human biospecimens in Africa and globally.[4]
Wits stipulates that approved biobanks should be registered with the SA Department of Health; however, there is currently no mechanism to do so. Regulatory bodies therefore need to develop policies and guidelines for biobanking in SA in order for the proper ethicolegal regulation of biobanking to occur.[2-4] A working group, comprising members from various academic institutions representing the interests of research and biobanking in SA, has been constituted to address these issues. The Bridging Biobanking and Biomedical Research across Europe and Africa (B3Africa) initiative is looking to align the biobanking frameworks in Africa with those already established in Europe, through the collaboration of their 11 partners.[8]
The SA National Health Research Ethics Council develops guidelines for health research ethics committees, which have the responsibility to regulate research involving human subjects. The Wits Human Research Ethics Committee (HREC) (Medical), which is further advised by the Wits BEC subcommittee, regulates biobanks that store samples for research purposes at the university.[2,4] The BEC was officially constituted in 2013, as Wits took the initiative to establish regulations at an institutional level for biobanking.[2,4] The BEC stipulates their requirements under either public- or private-sector biobanks. As the SBIMB biobank is neither of these, and functions as a small-scale academic biobank, the application process was challenging. Ethics approval (ref. no. M1403107) was granted in 2015, and is valid for 5 years, with the allowance for audits or visits from members of the BEC. The SBIMB biobank is the first academic biobank to be approved by the Wits HREC, and follows the approval of two commercial service provider biobanks in Johannesburg. Future requirements for accreditation with the SA National Accreditation Body[9] strengthen the need for the involvement of the government, medical and regulatory bodies and legislation to provide SA biobanks with the means for the equivalent certification that is available elsewhere.
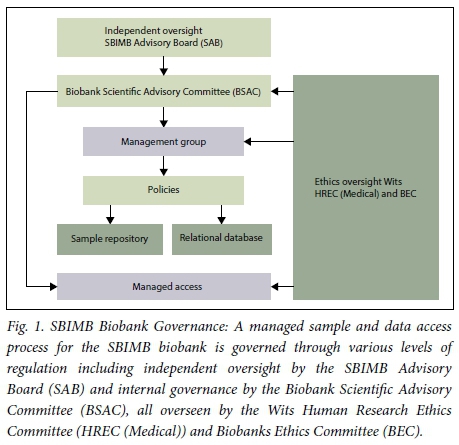
The SBIMB biobank has a governance structure for independent oversight through the SBIMB advisory board, which has both local and international representation. Internally, the Biobank Scientific Advisory Committee (BSAC) consists of a group of multidisciplinary individuals with a range of expertise, including clinicians, epidemiologists, geneticists, bioinformaticists and individuals with ethico-legal expertise. The BSAC manages access to both samples and associated data. Each request is assessed to ensure that ethical requirements have been met and that the research is in line with the informed consent provided by the participants, and the values and objectives of the SBIMB. The biobank management group is responsible for the daily running of the SBIMB biobank (Fig. 1). This system of governance ensures protection of the rights of research participants, regulates access to samples, and allows for future research in a manner that agrees with the ethical principles of beneficence, non-maleficence, respect for autonomy and justice, as well as being aligned with national and international guidelines and regulations which conform to the ideals of the Declaration of Taipei.
The Declaration of Taipei specifically focuses on the ethical considerations of biobanking.[10] It encompasses the collection, storage and use of human biospecimens beyond immediate healthcare and into the realm of public health.[10] This means that researchers are obliged to ensure that the rights of their participants are doubly protected.[10] Some informed-consent models are not adequate for biobanking, as they do not take into account the future use of participants' samples for unrelated research studies.[2,11] Broad consent allows ethical regulation of the use of biospecimens and data for research other than that stated in the original informed-consent document.[2-4,11] This implies that a strict governance framework is required to prevent misuse of stored samples and data, and to address the problems of exploitation and stigmatisation.[4,10,11] Community engagement and regular feedback on research outcomes are good ways to broaden understanding about research participation and its possible benefits to global health.[10,11]
All SBIMB biobank personnel are required to complete the National Institutes of Health (NIH) Protection of Human Participants online course annually, and to adhere to the policies and procedures outlined by the SBIMB, Wits,[12]the H3Africa consortium[13,14] and International Society for Biological and Environmental Repositories (ISBER).[15] The SBIMB biobank has no direct contact with the research participants whose samples are stored. The principal investigator (PI) of each research project storing samples in the biobank must provide a copy of the ethics clearance certificate, templates of the original informed-consent documents stating withdrawal options, an information sheet and an undertaking that all the donors of biospecimens submitted have signed consent forms. All samples are coded with a unique identifier that can only be linked to the participant by the project's PI, meaning that the SBIMB biobank has no access to personal identifying information. The minimum data accompanying each sample do not permit identification of participants, and related phenotypic, demographic and genomic data are not stored within the biobank, nor can they be accessed by unauthorised individuals. Details of consent are documented per study, and provision is made for specifying the use and sharing of samples and related data. The SBIMB biobank respects the rights of participants to withdraw from research projects. This is done following written instruction from the project PI for withdrawal and disposal of biological samples and data.
Infrastructure, sample management and storage
Location is an important factor to consider in academic research; researchers are more likely to make use of services if the biobank is easy to find and nearby, to limit the use of expensive couriers. Environment- and resource-specific factors may limit the feasibility of establishing a biobank, so it is advisable to consider the strengths and weaknesses of such a project.
In the case of the SBIMB biobank, potential modifications to the building were limited owing to the fact that the SBIMB is based on a national heritage site. The biobank itself is 70 m2 in size; therefore, only DNA and buffy coat samples are stored onsite, at -80°C. The size of the main laboratory, along with restrictions from the heritage council, prevents the installation of onsite CO2 backup cylinders, which presented a challenge due to the unstable power supply experienced in SA. To mitigate against the occasional loss of electricity, the SBIMB biobank runs on redundant power supply. In addition to the municipal supply, it has an uninterrupted power supply (UPS) with an extra battery, and a diesel generator capable of maintaining electricity levels for up to 48 hours per tank of diesel, and which can be topped up while running. It was necessary to consider site-specific modifications or risk preventive measures. For example, thunderstorms are common in Johannesburg in the summer and can cause power surges and outages, which necessitated the installation of earth mats and isolators. A biobank must have a back-up facility in close proximity that is capable of storing all samples in an emergency situation. The ambient temperature of the main laboratory and the temperatures of the fridges and freezers are monitored daily. The COMET temperature data monitoring system probes, which take temperature readings every 10 minutes, are linked to a modem that sends out text notifications at predetermined alarm-triggering temperatures, which for the ultra-low temperature freezers is -70°C.
Biobank personnel are trained to extract DNA from various sample types (whole blood, buffy coat, saliva, buccal swabs and stool samples). At least two aliquots of DNA are stored per participant. One aliquot is frozen at -80°C, while the other is refrigerated as a working aliquot. The working aliquot prevents multiple freeze-thaw cycle degradation of DNA. Ensuring high-quality DNA samples does not just rely on adequate storage, but may be affected by pre-analytical handling of samples (e.g. whole blood) from collection through to processing. A strict internal quality control (IQC) regimen is essential. An initial assessment of the quality of the samples received should be considered as part of the quality control (QC) process. Generally, whole blood and buffy coat samples that are clotted, or that have been stored inappropriately, perform poorly during automated extraction, because the tips or needles are unable to take up the sample. Their DNA yields are therefore much lower than the average expected yield for manual extractions in these cases (below 80 μg). DNA concentration and purity (260/280 ratio indicating protein contamination) should be assessed using spectrophotometry (Nanodrop, Thermo Fisher Scientific, USA), although fluorometric (Qubit, Life Technologies, Singapore) methods provide a more accurate measure of double-stranded DNA in a sample. Agarose gel electrophoresis should also be a standard requirement in QC to check DNA integrity. Running single nucleotide polymorphism (SNP) identification panels for each sample is recommended, but costly; therefore, in lower-resource settings a multiplex polymerase chain reaction assay for a small set of SNPs may be advised as a compromise. QC results are recorded in the laboratory information management system (LIMS), and monthly reports and other supporting documentation are stored electronically, in accordance with the quality management standards regarding document control outlined in the international standard for document storage (ISO 9001:2015).[16]According to ISO 15489-1:2001, records should be created, stored, maintained and protected such that their accuracy, legibility and availability are ensured.[17] Along with secure access and user verification, audit trails through electronic logs should be available to comply with best practice guidelines, ethics regulations and international standards.[17]
Laboratory information management system (LIMS)
Sample tracking and storage location management are key aspects of biobanking. The efficient management and tracking of samples as they are received, processed, aliquotted and shipped are best performed by the implementation of LIMS software. Data security is integral to the reliable and efficient management of samples and data in a biobank. The software should be secure, and access to data should only be granted to authorised users according to user-defined roles.
There are many commercial applications that offer comprehensive and fully auditable information and sample management, e.g. STARLIMS, Nautilus (ThermoScientific), CloudLIMS, LDMS and Gemini Matrix, to name a few. There are also software applications that only manage sample tracking, such as TD-Biobank and Freezerworks. Although these applications are fully integrated and supported, the costs may be prohibitive in a low-resource setting, as they may include annual licence fees, initial setup and customisation costs, and training by international companies. Choosing a LIMS depends on specific requirements, affordability and personnel. Open-source software (OSS) may be preferable if resources are not available to dedicate to a LIMS. Software such as Bika LIMS and the Ark Informatics can be customised to suit the needs of any biobank, provided that a dedicated developer can be employed for customisation, support and implementation. The cost-effectiveness of using an OSS package is balanced against the cost of the developer, the regular updating of the software by its creators, the customisability of the software to one's requirements, and the ease of implementation.
The Ark Informatics is a secure open-source LIMS software application developed within the Centre for Genetic Epidemiology and Biostatistics at the University of Western Australia in 2009.{18] It is module-based software that allows one to store minimum participant data, including data specific to consent and a full sample-location management system. It has the capacity to produce records upon request, allowing for a full audit trail. Samples received may be logged manually or according to bulk upload features. Depending on the project, the Ark Informatics can incorporate data from a database or from spreadsheets to organise all data specific to sample storage (Fig. 2). The SBIMB's version of the Ark was modified to suit our requirements, and allows automatic uploads from REDCap{19] or any other database in a seamless workflow. Despite initial implementation challenges, it is easy to use and has the relevant features that commercial systems offer.

The eB3Kit Biobank in a Box (Bibbox) (Austria), available through B3Africa, provides an integrated biobanking and bioinformatics platform.[8,20] This system consists of seven integrated work packages that monitor all aspects of biobanking, from the ethical and regulatory requirements, hardware and software requirements, a LIMS and bioinformatics platform, relevant training and dissemination and searchable datasets linked with biospecimens.[20] Once the eBiokit is purchased, the software is free and tools are available in the Bibbox ecosystem for personal customisation.[8,20] Currently Bika is being implemented, but support will also be available for Open Specimen.[8,20] Other LIMS can be hooked up to the eBiokit for bioinformatics analysis (Galaxy/Pulsar), experiment management, Open Data Kit (ODK) for data collection with mobile phones and ethics compliance support.[8,20] Had it been available when we started the SBIMB biobank, it would have been evaluated and considered before making a decision on the most suitable LIMS option.
Discussion
The SBIMB biobank houses a valuable and constantly growing resource for multi-disciplinary research in SA, and aims to be sustainable in the long term. Our main objective is to provide a high-quality bioresource, as well as expertise to support analysis of data and samples from African populations, so that we can better characterise, understand and apply our research findings to improve the health of Africans. The establishment of a national biobank in SA has been under discussion since 2013.[1,2] Lack of adequate financial resources and insufficient current local investment in national research infrastructure, as well as misconceptions regarding ownership of biospecimens, have to date confounded the development of a feasible model for a national biobank in SA. Developing sustainability models for biobanks in low-resource settings is essential, as the funds required to run a biobank on an annual basis are significant (Table 1). In accordance with best-practice guidelines, contingency plans should be considered in the event of possible closure precipitated by a lack of sustained funding.[2,3] To mitigate such risk, support from large institutions (e.g. universities or the government), and the introduction of a 'pay-for-services' culture between collaborators and biobank users, would support sustainability. The SBIMB biobank has implemented a business model where researchers using the services pay according to their required needs, including the costs for the reagents and consumables used for their projects, personnel time and an annual storage fee.
Academic researchers from various universities in SA have initiated research into the feasibility of institution-based biobanks operating with a standard set of guidelines and an agreed-upon set of standard operating procedures regarding sample and data sharing, sample handling and QC. Perhaps the most feasible outcome for SA is the implementation of a virtual national network of collaborating biobanks, working according to harmonised standards and procedures. Developing a website listing the biobanks in SA, and cataloguing their respective studies and samples, will promote further collaboration, and will identify synergies to bolster national research efforts.
Establishing the SBIMB biobank has provided insight into important considerations that should be addressed in the planning stages of setting up an academic biobank. These include: necessity and feasibility (do you need to start your own biobank or can you use existing facilities?); ethical, legal and social considerations of setting up a biobank (governance, legislation and regulation); location; infrastructure (fit for purpose with suitable back-up strategies); storage (what sample types are being stored and at what temperature?); LIMS (commercial v. open source packages); accreditation or approval; and sustainability (have you developed a sustainable funding model?). Each of these considerations has been addressed in this article not only in terms of biobanking in general, but in an SA context, specifically in an academic setting. In Table 2 we provide ten broad areas for consideration when planning to set up a biobank. We suggest key questions that should be addressed, and outline minimum requirements for a successful biobanking endeavour.
Setting up a biobank requires commitment, funding, institutional support, a team of dedicated researchers and considerable time. It took 3 years for the SBIMB biobank to be ready to process, store and provide high-quality DNA samples for academic research, and to obtain approval from the Wits HREC (Medical) (Fig. 3). We have plans to expand the SBIMB biobank in order to increase our research and storage capacity, and to promote further cross-institutional and cross-disciplinary biomedical research.
Acknowledgements. Research reported in this publication was supported by the National Human Genome Research Institute of the National Institutes of Health under Award Number U54HG006938, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Office of the Director, National Institutes of Health. MR is an SA Research Chair in Genomics and Bioinformatics of African populations hosted by Wits, funded by the Department of Science and Technology and administered by the National Research Foundation of SA. Wits provided infrastructure support for the Biobank. We thank the repository team from Clinical Laboratory Services, especially Dr Ute Jentsch for her invaluable advice and for assisting us in preparing our documents during the ethics application process. We thank Wits for providing us with infrastructure and general services, and the Wits BEC for their guidance and approval. A special thanks to Dr Zané Lombard and Dr Nadia Carstens for their contributions at the initial planning stages, and to Dr Carmen Swanepoel and Dr Micheline Sanderson for their expert advice. Lastly, thank you to the SBIMB biobank personnel (Natalie Smyth and Sebentile Hleli Mthimkulu) for their continuing hard work.
References
1. Abayomi A, Christoffels A, Grewal R, et al. Challenges of biobanking in South Africa to facilitate indigenous research in an environment burdened with human immunodeficiency virus, tuberculosis, and emerging noncommunicable diseases. Biopreserv Biobank 2013;11(6):347-354. https://doi.org/10.1089/bio.2013.0049 [ Links ]
2. Dhai A. Establishing national biobanks in South Africa: The urgent need for an ethicoregulatory framework. S Afr J Bioeth Law 2013;6(2):38-39. https://doi.org/10.7196/SAJBL.296 [ Links ]
3. Dhai A, Mohamed, S. Biobank research: Time for discussion and debate. S Afr Med J 2013;103(4):225-227. https://doi.org/10.7196/SAMJ.6813 [ Links ]
4. Mahomed S, Behrens K, Slabbert M, Sanne I. Managing human tissue transfer across national boundaries - an approach from an institution in South Africa. Dev World Bioeth 2016;16(1):29-35. https://doi.org/10.1111/dewb.12080 [ Links ]
5. South Africa. National Health Act No. 61 of 2003. Regulations: General control of human bodies, tissue, blood, blood products and gametes. Government Gazette No. 35099, 2012. (Published under Government Notice R180. [ Links ])
6. De Vries J, Pepper M. Genomic sovereignty and the African promise: Mining the African genome for the benefit of Africa. J Med Ethics 2012;38(8):474-478. https://doi.org/10.1136/medethics-2011-100448 [ Links ]
7. University of the Witwatersrand: Steve Biko Centre for Bioethics. Material Transfer Agreement for Human Biological Materials. Johannesburg: Wits, 2015. http://www.wits.ac.za/research/about-our-research/ethics-and-research-integrity/biobanks-ethics-committee (accessed 17 August 2015). [ Links ]
8. Bridging biobanking and biomedical research across Europe and Africa. Bridging Biobanking and Biomedical Research across Europe and Africa Newsletter. B3A, 2016: 1. http://www.b3africa.org (accessed 11 May 2016). [ Links ]
9. South African National Accreditation System. South Africa: SANAS, 2015. http://www.home.sanas.co.za (accessed 24 May 2016). [ Links ]
10. World Medical Association Inc. WMA Declaration of Taipei on Ethical Considerations regarding Health Databases and Biobanks. France: WMA, 2016. http://www.wma.net/en/30publications/10policies/d1/ (accessed 12 January 2017). [ Links ]
11. Tindana P, de Vries J. Broad consent for genomic research and biobanking: Perspectives from low- and middle-income countries. Annu Rev Genomics Hum Genet 2016;17:375-393. https://doi.org/10.1146/annurev-genom-083115-022456 [ Links ]
12. University of the Witwatersrand: Steve Biko Centre for Bioethics. Biobanks Ethics Committee of the University of the Witwatersrand's HREC (Medical). The Human Research Ethics Committee Medical (HREC) Principles and Policy on Biobanks. Johannesburg: Wits, 2015. http://www.wits.ac.za/research/about-our-research/ethics-and-research-integrity/biobanks-ethics-committee (accessed 17 August 2015). [ Links ]
13. H3Africa Consortium. H3Africa Working Group on Ethics and Regulatory Issues for Human Heredity and Health in Africa Consortium. H3Africa Guidelines for Informed Consent, Second Edition. H3A, 2014. http://www.h3africa.org/images/GuidelinesPolicyDocs/CE%20Guidelines_Final.pdf (accessed 30 May 2016). [ Links ]
14. H3Africa Consortium. H3Africa Working Group on Data Sharing, Access and Release for the Human Heredity and Health in Africa Consortium. H3Africa Consortium Data Sharing, Access and Release Policy. H3A, 2014. http://www.h3africa.org/images/DataSARWG_folders/FinalDocsDSAR/H3Africa%20Cons ortium% 20Data%20Access%20%20Release%20Policy%20Aug%202014.pdf (accessed 30 May 2016). [ Links ]
15. Astrin J, Baker S, Barr TJ, et al. Best practices for repositories collection, storage, retrieval, and distribution of biological materials for research. Biopreserv Biobank 2012;10(2):79-161. https://doi.org/10.1089/bio.2012.1022 [ Links ]
16. South African Bureau of Standards. SANS 9001:2015 ISO 9001:2015, 5th ed. South African National Standard - Quality Management Systems Requirements. SABS: Pretoria, 2015. [ Links ]
17. South African Bureau of Standards. SANS 15489-1:2004 ISO 15489-1:2001, 1st ed. South African National Standard - Information and Documentation Records management. SABS: Pretoria, 2004 [ Links ]
18. Nectar. Australia: National Research Infrastructure for Australia, 2016. https://www.nectar.org.au (accessed 10 November 2015). [ Links ]
19. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010 [ Links ]
20. Klingström T, Mendy M, Meunier D, et al. Supporting the Development of Biobanks in Low and Medium Income Countries. IST-Africa 2016 Conference Proceedings, 2016. https://doi.org/10.1109/ISTAFRICA.2016.7530672 [ Links ]
 Correspondence:
Correspondence:
M Ramsay
michele.ramsay@wits.ac.za
Accepted 20 February 2017














