Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.107 n.5 Pretoria May. 2017
http://dx.doi.org/10.7196/samj.2017.v107i5.10692
RESEARCH
Bacteria isolated from the airways of paediatric patients with bronchiectasis according to HIV status
C Verwey; S Velaphi; R Khan
FCPaed (SA) Department of Paediatrics, Chris Hani Baragwanath Academic Hospital and Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
BACKGROUND. Knowledge of which bacteria are found in the airways of paediatric patients with bronchiectasis unrelated to cystic fibrosis (CF) is important in defining empirical antibiotic guidelines for the treatment of acute infective exacerbations.
OBJECTIVE. To describe the bacteria isolated from the airways of children with non-CF bronchiectasis according to their HIV status.
METHODS. Records of children with non-CF bronchiectasis who attended the paediatric pulmonology clinic at Chris Hani Baragwanath Academic Hospital, Johannesburg, South Africa, from April 2011 to March 2013, or were admitted to the hospital during that period, were reviewed. Data collected included patient demographics, HIV status, and characteristics of the airway samples and types of bacteria isolated.
RESULTS. There were 66 patients with non-CF bronchiectasis over the 2-year study period. The median age was 9.1 years (interquartile range 7.2 - 12.1). The majority of patients (78.8%) were HIV-infected. A total of 134 samples was collected (median 1.5 per patient, range 1 - 7), of which 81.3% were expectorated or induced sputum samples. Most bacteria were Gram negatives (72.1%). Haemophilus influenzae was the most common bacterium identified (36.0%), followed by Streptococcus pneumoniae (12.6%), Moraxella catarrhalis (11.1%) and Staphylococcus aureus (10.6%). There were no differences between HIV-infected and uninfected patients in prevalence or type of pathogens isolated.
CONCLUSION. Bacterial isolates from the airways of children with non-CF bronchiectasis were similar to those in other paediatric populations and were not affected by HIV status.
Bronchiectasis is a chronic suppurative pulmonary disorder generally defined as irreversible dilatation of the central and peripheral airways.[1] It is associated with periodic infectious exacerbations, which are defined as an increase in the severity and wet character of the cough.[2] There is chronic bacterial colonisation of the bronchi, and an increase in inflammatory cells and mediators in the bronchial mucosa and bronchial walls.[3] This leads to a vicious cycle of recurrent chest infections and chronic inflammatory changes leading to the eventual destruction of the structural elements within the bronchial wall.
Irrespective of the cause of the bronchiectasis, the structural damage to the airways predisposes the patient to repeated lower respiratory tract infections that can cause further damage to the lungs.[4] To limit damage to the airways and lung parenchyma, acute infective exacerbations should be treated early and aggressively with antimicrobials. Knowing which bacteria are found in the airways of patients with bronchiectasis is important in defining empirical antibiotic guidelines for the management of acute infective exacerbations.
The airway-colonising bacteria resulting in acute infective exacerbations in patients with cystic fibrosis (CF) bronchiectasis have been extensively described. Colonisation takes place from an early age, with Staphylococcus aureus and Haemophilus influenzae being common, followed by Pseudomonas aeruginosa, which becomes increasingly prominent in older patients.[5]
Studies in adult patients with non-CF bronchiectasis have shown that H. influenzae, P. aeruginosa and Streptococcus pneumoniae are the most common organisms isolated.[6] Adult patients with CF bronchiectasis are more likely to be colonised with P. aeruginosa than adults with non-CF bronchiectasis.[7]
An increasing number of studies have described the organisms colonising the airways in paediatric non-CF bronchiectasis.[8] The bacteria most commonly found to be colonising the airways of HIV-uninfected children with non-CF bronchiectasis are H. influenzae, S. pneumoniae, Moraxella catarrhalis and S. aureus. Most of these studies are from developed countries where there have been no reports on bacteria colonising the airways of HIV-infected patients. In developing countries, Ferrand et al.[9]described the organisms found in the airways of adolescents with vertically transmitted HIV in Zimbabwe. A positive bacterial/fungal sputum culture was reported in 18 of 54 patients with cough, H. influenzae (n=6), S. aureus (n=5), M. catarrhalis (n=5), P. aeruginosa (n=3) and S. pneumoniae (n=2) being the most commonly reported bacteria. Masekela et al.[10] studied a cohort of 35 HIV-infected children with bronchiectasis in South Africa (SA), and found that the most common organisms were H. influenzae and H. parainfluenzae, which accounted for 49% of organisms cultured.
It is not clear whether bacterial colonisation of the airways in non-CF bronchiectasis differs between HIV-infected and HIV-uninfected children. Having this information is vital when choosing which antimicrobials to use during an acute exacerbation.
Objective
To determine the bacteria isolated from children with non-CF bronchiectasis in a setting with a high prevalence of HIV.
Methods
Study design
This was a retrospective observational study.
Study setting
The study setting was Chris Hani Baragwanath Academic Hospital (CHBAH), a tertiary public government hospital in Soweto, Johannesburg, SA. All paediatric patients with suspected bronchiectasis are referred to a paediatric pulmonologist or to the paediatric pulmonology clinic. Diagnosis of bronchiectasis is made on clinical assessment and confirmed with a chest radiograph or a computed tomography scan of the chest.
Study population
The study population comprised children aged <16 years attending the paediatric pulmonology clinic or admitted to CHBAH with a diagnosis of non-CF bronchiectasis from 1 April 2011 to 31 March 2013. Children with bronchiectasis in whom CF had been confirmed were excluded from the study.
Management of patients with bronchiectasis at CHBAH
Airway samples were taken for bacterial surveillance from all patients at the time of diagnosis of bronchiectasis. Samples were then taken on a regular basis, approximately every 3 months, and during every acute exacerbation or admission. Airway samples were collected by spontaneous expectoration, sputum induction, tracheal aspiration (TA) or bronchoalveolar lavage (BAL) during bronchoscopy. All samples were sent to the National Health Laboratory Service (NHLS) at CHBAH.
All samples were first examined with the Gram staining method and designated Gram-positive or negative. Qualitative tests were performed to determine whether the samples taken were representative of the upper or lower respiratory tract by assessing the number of polymorphonuclear and squamous epithelium cells per low-power field (10x magnification). Only samples from the lower airways were included in the analysis. Samples were inoculated on 5% horse blood agar incubated in CO2 at 35oC, MacConkey agar incubated aerobically at 35oC, and bacitracin heated blood agar incubated in CO2 at 35oC. All inoculated media were read after 24 and 48 hours. The bacterial organism was identified if any of the plates yielded positive growth. Positive cultures were further quantified to establish the density of the bacterial growth in the culture. Culture density was reported as <10 colony-forming units (CFU), 10 - 99 CFU or >100 CFU on the initial inoculum. Specific anaerobic cultures were not routinely performed owing to the technical difficulties and low yield of this test.
HIV testing was routinely performed on all patients admitted to the paediatric wards according to National Department of Health guidelines. HIV serological testing using the enzyme-linked immunosorbent assay (ELISA) was carried out for children >18 months of age, with a confirmatory serological test (HIV combination Ab/P24 Ag) performed if the ELISA was reactive. HIV polymerase chain reaction (qualitative) testing was used for children aged <18 months.
Data collection
Data were collected from the patients' paediatric pulmonology files and from the computerised NHLS results database. The following data were collected: patient demographics, past medical history, radiological features, and laboratory findings including HIV status, CD4 count, viral load, number and type of sputum samples collected, sputum microscopy and sputum culture results.
Data analysis
All samples collected from patients were included in the analysis, including multiple samples from the same patient. Data were entered onto an Excel spreadsheet, version 2010 (Microsoft, USA). Statistical analysis was performed using Statistica version 12 (StatSoft, USA). Data were summarised using means and standard deviations (SDs) for continuous variables with normal distribution; medians, interquartile ranges (IQRs) and ranges for continuous data without normal distribution; and proportions and percentages for categorical variables. Comparisons between HIV-infected and uninfected patients with bronchiectasis were performed using Student's f-test for continuous variables with normal distribution and the MannWhitney (7-test for those without normal distribution. The χ2 test or Fisher's exact test was used for categorical variables. Differences were considered to be statistically significant when the p-value was <0.05.
Ethics approval
Permission to perform the study was obtained from the Hospital Protocol Review Committee and the Human Research Ethics Committee of the University of the Witwatersrand (ref. no. M130415).
Results
Sixty-six patients with non-CF bronchiectasis had airway samples analysed at CHBAH from April 2011 to March 2013 (Table 1). Most patients were of black African descent (96.9%), and 78.8% of patients were HIV-infected. The median CD4 percentage and viral load in the HIV-infected patients were 17.2% (IQR 4.7 - 28.5) and 18 422 copies/ mL (IQR 0.0 - 166 475), respectively. Thirty-seven HIV-infected patients (71.1%) were known to be on antiretrovirals (ARVs), and in 30 patients the duration of ARV therapy was known (median duration 60 months, IQR 12 - 84). There was no statistically significant difference between the HIV-infected and uninfected patients with regard to gender (54.5% female). HIV-infected patients were older at presentation (median age 11.4 years, IQR 7.7 - 12.5) than HIV-uninfected children (median age 7.1 years, IQR 6.0 - 7.8) (p=0.002).
Sixty-three percent of patients had bilateral bronchiectasis, with the mean (SD) number of lung lobes affected being 3 (1.4). There was no difference in this regard between HIV-infected and uninfected patients. The underlying causes of the bronchiectasis in the HIV-uninfected group were post-infectious (n=5), post-tuberculosis infection (n=1), primary immunodeficiency (n=3), non-HIV acquired immunodeficiency (malignancy and chemotherapy) (n=1), aspiration syndrome (n=3) and unknown (n=1). A total of 134 samples were collected from the 66 patients (Table 2). The median number of samples collected per patient was 2 (range 1 - 7). Thirty-three patients (50.0%) had 1 sample collected, 17 (25.8%) had 2 samples collected and 16 (24.2%) had >3 samples collected. There was no statistical difference between the number of samples collected from HIV-infected and uninfected patients. Most specimens (81.3%) were collected as expectorated or induced sputum; only 25 were BAL or TA specimens. More HIV-uninfected patients than HIV-infected patients had BAL specimens collected (p<0.05) (Table 2).
There was no statistical difference between HIV-infected and HIV-uninfected patients when comparing the number of positive and negative bacterial cultures, bacteria cultured per sample or the density of individual bacterial culture colonies (Table 2).
The main bacteria cultured were H. influenzae, S. pneumoniae, M. catarrhalis, S. aureus, Pseudomonas species, Klebsiella species and H. parainfluenzae (Fig. 1).
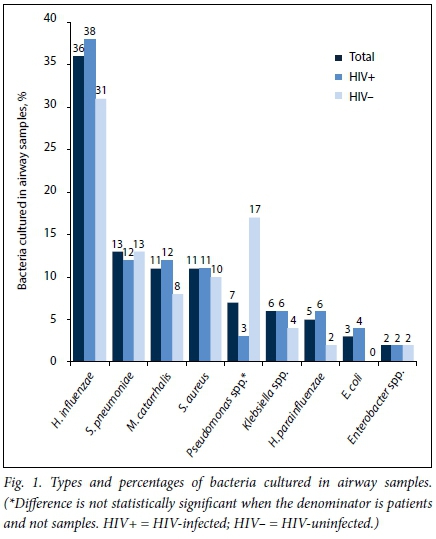
The types and numbers of Gram-negative and Gram-positive bacteria cultured in all samples are shown in Table 3.
When comparing samples from HIV-infected and uninfected patients with regard to bacteria cultured, there were no statistically significant differences except that Pseudomonas species were more likely to be cultured from HIV-uninfected patient samples (Table 3). When reanalysing the data on an individual patient basis, it was found that one patient had seven samples positive for P. aeruginosa, and there was no statistical difference between HIV-infected and uninfected patients in frequency of P. aeruginosa in airway samples.
Thirty-three patients (50.0%) had two or more samples collected, and of these 28 (84.8%) cultured at least one bacterium in two or more samples. Of these, 20 (71.4%) had recurrence of the same organism in subsequent samples. When H. influenzae was cultured in a sample from an individual patient, there was a 69.5% chance that it would be cultured in subsequent samples from the same patient. There was a 63.6% chance of this occurring for S. pneumoniae, a 50.0% chance for M. catarrhalis and a 25.0% chance for S. aureus. H. parainfluenzae was not cultured in subsequent sputum samples for any individual patient in this study.
When looking at the number of individual patients who had specific bacteria cultured at any stage there were no significant differences between HIV-infected and uninfected patients for any of the main bacteria cultured (H. influenzae, S. pneumoniae, M. catarrhalis, S. aureus, H. influenzae, Pseudomonas species and Klebsiella species).
Discussion
The objective of this study was to determine whether there are differences in the number and type of bacteria cultured from the airways of HIV-infected and uninfected children.
The majority of patients with bronchiectasis in our study were HIV-infected, with active disease as shown by low CD4 counts and high viral loads. The most common bacteria cultured from their airways were H. influenzae, S. pneumoniae, M. catarrhalis and S. aureus. When the bacteria cultured from the airways of HIV-infected and uninfected children were compared, there were no differences in the number of bacterial species cultured per airway sample or the type of bacteria cultured.
In this study, over three-quarters (84.3%) of specimens had at least one bacterium cultured, with 46.2% culturing two or more types of bacteria. The high rate of positive samples is similar to most reports from countries with a low HIV incidence,[11-13] in contrast to Karadag et al.,[14] who isolated bacteria in only 46.9% of patients with bronchiectasis. The reason for this difference is not clear, but it could be related to the age at presentation of the patients, the severity of the bronchiectasis and the fact that the majority of our patients were HIV-infected, though we did not find differences in type and number of bacteria cultured between HIV-infected and uninfected patients. The mean (SD) age of the patients in the Turkish cohort was 7.4 (3.7) years at presentation, while the mean age of our patients was 9.7 (3.3) years. This would allow more time for untreated patients to acquire more acute infective exacerbations and possibly to be colonised by a larger number of bacterial species. Ferrand et al.[9] reported on a cohort of adolescents with HIV-associated lung disease and reported positive bacterial cultures in only 33% of their patients. The low rate of positive cultures in their cohort is probably because while all the children in their study had HIV-associated chronic lung disease, only 43% had bronchiectasis, whereas all the patients in our study had bronchiectasis. Masekela et al.,[10] in a study from SA, reported a positive culture rate of 65% when cumulative samples over a 1-year period were included.
H. influenzae was the most common bacterium reported in all studies, with a frequency ranging between 31% and 68%; and the frequency in our study was 36.0%. The majority of studies have reported similar results in terms of the most common bacteria cultured, namely H. influenzae followed by S. pneumoniae, M. catarrhalis and S. aureus.[12,15] Other studies have reported common bacteria that vary slightly from this study, with Karadag et al.[14]and Li et al.[13]reporting, in order of declining frequency, H. influenzae, S. pneumoniae, S. aureus and P. aeruginosa. The bacteria reported by these authors are similar to those in our study, except for a higher frequency of P. aeruginosa in their cohorts. The reason for this finding is not clear, but it was reported by Kapur et al.[11]that the presence of P. aeruginosa colonisation of the airways of the non-CF child with bronchiectasis could signal the presence of a serious underlying co-morbidity. Indeed, the only case of P. aeruginosa in the airway of a child without CF found by Edwards et al.[16]was in a patient with a tracheostomy.
Masekela et al.[10]reported that the most commonly cultured bacteria in samples from children who were HIV-infected and had bronchiectasis were H. influenzae (30%) and H. parainfluenzae (21%), with M. catarrhalis following at 4% and no S. pneumoniae, S. aureus or P. aeruginosa reaching a frequency of >2%. These findings are very different to ours, with a much higher frequency of H. parainfluenzae and much lower frequencies of all the other bacteria. The reason for this difference is unclear. The findings of Ferrand et al.[9] were similar to ours, but with a lower frequency of S. pneumoniae and a higher frequency of S. aureus. The numbers in the Ferrand study were very small and therefore difficult to interpret.
Study limitations
There are a number of limitations to this study. Firstly, it was a retrospective observational study, potentially allowing for selection and information bias. It is possible that not all patients with bronchiectasis were referred to the paediatric pulmonology service. Secondly, the number of HIV-uninfected patients was quite small compared with the number of HIV-infected patients, and owing to the retrospective nature of the study they were not perfectly matched with the HIV-infected patients, especially with regard to age. There is also current evidence that the lung microbiome in HIV-infected patients with low CD4 counts is different to that in patients with preserved CD4 counts, and that even after highly active antiretroviral therapy these changes are not fully reversed.[17] In theory, this could also have influenced the results of this study. More HIV-uninfected than infected patients had BAL samples taken, which may have influenced the outcome of the culture results. HIV-uninfected children are more likely to have undergone bronchoscopy in the course of work-up for the cause of their bronchiectasis, while in HIV-infected children the cause of the bronchiectasis is usually known to be recurrent and severe chest infections. BAL samples are collected from a specific area in the lung, usually determined as the most representative area of disease on radiological assessment, while in theory an expectorated sample is more representative of the entire lung. This may also have influenced the findings on culture of the samples. Another limitation is that it was not often recorded whether samples were collected during acute infective exacerbations or routine clinic follow-up visits. There is a possibility that sputum microflora could differ between routine collections and times of acute infective exacerbations, although this has not been shown in previous studies, and the recommendation is still to treat acute infective exacerbations with antibiotics that are guided by the bacteria cultured during healthy periods.[18]
Although there were fewer HIV-uninfected than HIV-infected patients, a strength of this study is that we made a direct comparison of numbers and types of bacteria grown in the samples from airways of HIV-infected and uninfected patients, which has not been reported in previous studies.
Conclusion
Antibiotics prescribed for an acute exacerbation in children with non-CF bronchiectasis, regardless of HIV status, should be targeted against Gram-positive and Gram-negative bacteria including H. influenzae, S. pneumoniae and S. aureus.
Acknowledgements. This research report was submitted to the Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, in partial fulfilment of the requirements for the MMed (Paediatrics). The research was accepted as an abstract and presented as a poster at the International Congress on Paediatric Pulmonology (CIPP 1), 25 - 28 June 2015, Krakow, Poland.
Author contributions. CV conceptualised and designed the study, collected all data, analysed all data, interpreted the data and drafted the manuscript. SV and RK contributed to drafting and revising the manuscript. All authors gave final approval for the manuscript to be published.
Funding. None.
Conflicts of interest. None.
References
1. Field CE. Bronchiectasis in childhood: Clinical survey of 160 cases. Pediatrics 1949;4(1):21-46. [ Links ]
2. Kapur N, Masters IB, Chang AB. Exacerbations in noncystic fibrosis bronchiectasis: Clinical features and investigations. Resp Med 2009;103(11):1681-1687. http://dx.doi.org/10.1016/j.rmed.2009.05.007 [ Links ]
3. Gaga M, Bentley AM, Humbert M, et al. Increases in CD4+ T lymphocytes, macrophages, neutrophils and interleukin 8 positive cells in the airways of patients with bronchiectasis. Thorax 1998;53(8):685-691. http://dx.doi.org/10.1136/thx.53.8.685 [ Links ]
4. Cole P. The damaging role of bacteria in chronic lung infection. J Antimicrob Chemother 1997;40(Suppl A):5-10. http://dx.doi.org/10.1093/jac/40.suppl_L5 [ Links ]
5. Hauser AR, Jain M, Bar-Meir M, McColley SA. Clinical significance of microbial infection and adaptation in cystic fibrosis. Clin Microbiol Rev 2011;24(1):29-70. http://dx.doi.org/10.1128/CMR.00036-10 [ Links ]
6. Angrill J, Agusti C, de Celis R, et al. Bacterial colonisation in patients with bronchiectasis: Microbiological pattern and risk factors. Thorax 2002;57(1):15-19. http://dx.doi.org/10.1136/thorax.57.1.15 [ Links ]
7. Athanazio RA, Rached SZ, Rohde C, Pinto RC, Fernandes FL, Stelmach R. Should the bronchiectasis treatment given to cystic fibrosis patients be extrapolated to those with bronchiectasis from other causes? J Bras Pneumonol 2010;36(4):425-431. http://dx.doi.org/10.1590/S1806-37132010000400006 [ Links ]
8. Grimwood K. Airway microbiology and host defences in paediatric non-CF bronchiectasis. Paediatr Respir Rev 2011;12(2):111-118. http://dx.doi.org/10.1016/j.prrv.2010.10.009 [ Links ]
9. Ferrand RA, Desai SR, Hopkins C, et al. Chronic lung disease in adolescents with delayed diagnosis of vertically acquired HIV infection. Clin Infect Dis 2012;55(1):145-152. http://dx.doi.org/10.1093/cid/cis271 [ Links ]
10. Masekela R, Anderson R, Moodley T, et al. HIV-related bronchiectasis in children: An emerging spectre in high tuberculosis burden areas. Int J Tuberc Lung Dis 2012;16(1):114-119. http://dx.doi.org/10.5588/ijtld.11.0244 [ Links ]
11. Kapur N, Grimwood K, Masters IB, Morris PS, Chang AB. Lower airway microbiology and cellularity in children with newly diagnosed non-CF bronchiectasis. Pediatr Pulmonol 2012;47(3):300-307. http://dx.doi.org/10.1002/ppul.21550 [ Links ]
12. Eastham KM, Fall AJ, Mitchell L, Spencer DA. The need to redefine non-cystic fibrosis bronchiectasis in childhood. Thorax 2004;59(4):324-327. http://dx.doi.org/10.1136/thx.2003.011577 [ Links ]
13. Li AM, Sonnappa S, Lex C, et al. Non-CF bronchiectasis: Does knowing the aetiology lead to changes in management? Eur Respir J 2005;26(1):8-14. http://dx.doi.org/10.1183/09031936.05.00127704 [ Links ]
14. Karadag B, Karakoc F, Ersu R, Kut A, Bakac S, Dagli E. Non-cystic-fibrosis bronchiectasis in children: A persisting problem in developing countries. Respiration 2005;72(3):233-238. http://dx.doi.org/10.1159/000085362 [ Links ]
15. Edwards EA, Asher MI, Byrnes CA. Paediatric bronchiectasis in the twenty-first century: Experience of a tertiary children's hospital in New Zealand. J Paediatr Child Health 2003;39(2):111-117. http://dx.doi.org/10.1046/j.1440-1754.2003.00101.x [ Links ]
16. Edwards EA, Metcalfe R, Milne DG, Thompson J, Byrnes CA. Retrospective review of children presenting with non cystic fibrosis bronchiectasis: HRCT features and clinical relationships. Pediatr Pulmonol 2003;36(2):87-93. http://dx.doi.org/10.1002/ppul.10339 [ Links ]
17. Twigg HL 3rd, Weinstock GM, Knox KS. Lung microbiome in human immunodeficiency virus infection. Transl Res 2017;179:97-107. http://dx.doi.org/10.1016/j.trsl.2016.07.008 [ Links ]
18. Pasteur MC, Bilton D, Hill AT. British Thoracic Society guideline for non-CF bronchiectasis. Thorax 2010;65(Suppl 1):i1-i58. http://dx.doi.org/10.1136/thx.2010.136119 [ Links ]
 Correspondence:
Correspondence:
C Verwey
charl.verwey@wits.ac.za
Accepted 24 January 2017














