Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.107 n.4 Pretoria Apr. 2017
http://dx.doi.org/10.7196/samj.2017.v107i4.12198
RESEARCH
Prevalence of hepatitis B, hepatitis C and human immunodeficiency viruses, and evaluation of risk factors for transmission: Report of a population screening in Nigeria
U C OkonkwoI; H OkparaII; A OtuIII; S AmehIV; Y OgarekpeV; H OsimVI; M InyamaVII
IMD, FWACP, FMCP; Gastroenterology/Hepatology Unit, Department of Internal Medicine, University of Calabar Teaching Hospital, Cross River State, Nigeria
IIMD, MPH, FMCPath; Department of Chemical Pathology, University of Calabar Teaching Hospital, Cross River State, Nigeria
IIIMD, MPH, FWACP; Infectious Disease Unit, Department of Internal Medicine, University of Calabar Teaching Hospital, Cross River State, Nigeria
IVMD, MPH, FMCPH, FWACP; Department of Community Medicine, University of Calabar Teaching Hospital, Cross River State, Nigeria
VMD; Department of Chemical Pathology, University of Calabar Teaching Hospital, Cross River State, Nigeria
VIMD; Gastroenterology/Hepatology Unit, Department of Internal Medicine, University of Calabar Teaching Hospital, Cross River State, Nigeria
VIIMD, FMCPath; Department of Haematology, University of Calabar Teaching Hospital, Cross River State, Nigeria
ABSTRACT
BACKGROUND. Hepatitis B virus (HBV), hepatitis C virus (HCV) and HIV are common blood-borne infections unevenly distributed across regions in Nigeria. Few population-based prevalence studies have been done in Nigeria.
OBJECTIVE. To determine the prevalence of HBV, HCV and HIV and risk factors for infection with these viruses in a Nigerian population.
METHODS. Hepatitis B surface antigen, anti-HCV and HIV were assayed in 1 498 healthy adult participants. A structured questionnaire was used to assess risk factors for viral acquisition. Bivariate analysis was used to compare differences in sociodemographic characteristics. Significant risk factors were identified by stepwise logistic regression. A p-value <0.05 was considered significant.
RESULTS. The prevalences of HBV, HCV and HIV were 8.8%, 10.0% and 12.9%, respectively, with urban/rural disparity. HBV/HCV positivity was higher among males than females. The reverse was true for HIV. Age was significantly associated with being HBV-, HCV- or HIVpositive. Communal use of a toothbrush was significantly associated with HBV positivity in the final model (odds ratio 2.46, 95% confidence interval 1.45 - 4.18).
CONCLUSIONS. The prevalence of HBV, HCV and HIV infection is high in Nigeria, with urban/rural disparity. HCV may be more of a public health concern than HBV in some communities. Population-based studies are required to provide vital data to inform optimal national control strategies.
Hepatitis B virus (HBV), hepatitis C virus (HCV) and HIV are blood-borne infections that share common routes of transmission. The main modes of transmission of HBV and HIV are from mother to child, horizontal in early childhood, and from unprotected sexual contact, sharing of sharps and injection drug abuse. HCV is transmitted mostly through direct contact with the blood of an infected person via blood transfusion and intravenous drug use.[1] The prevalence of mono-infection and co-infection is therefore common, especially in low- and middle-income countries in South-East Asia and Africa.[2,3]
Globally, it is estimated that 2 billion persons have previous or current evidence of HBV infection, of whom 400 million are chronically infected.[4] HCV and HIV affect 170 million and 33 million persons, respectively.[3] Of the 33 million people living with HIV worldwide, 5 - 20% have chronic HBV infection and 5 - 15% hepatitis C infection, although the rate of hepatitis C infection may rise to 90% among people who inject drugs.[1] Of people living with HIV in the USA, ~25% are co-infected with HCV and ~10% are co-infected with HBV.[5]
Nigeria is hyperendemic for HBV, with a prevalence of 12%, but has a relatively low prevalence of HCV of 0.5 - 4% and a 3.1% prevalence of HIV. However, with a population of 170 million according to the 2006 census, this translates to approximately 23 million of its general population living with HBV, 1 - 6 million living with HCV and 3.5 million living with HIV.[6-8] The consequences of infection with these viruses, including hepatocellular carcinoma and liver cirrhosis, are responsible for >20% of mortality in Nigeria.[9] Cross River State (CRS), located in the Niger delta region of Nigeria, is thought to have among the highest prevalence rates of HIV, HBV and HCV in the country.[10,11]
Treatment options for individuals with chronic hepatitis B, C or HIV infection have improved considerably in the last few years. For HBV and HIV, the use of nucleoside or nucleotide analogues in addition to protease inhibitors (for HIV) leads to suppression of the virus in most patients and prevents transmission and onset of complications.[12] For HCV, the discovery of direct antiviral agents has transformed the disease from an incurable to a potentially curable condition with sustained virological response of >90% even in patients with advanced liver disease.[13,14] Bearing in mind the benefits of these treatment options, it is imperative to identify individuals who are infected with these viruses by providing effective screening programmes, and to initiate therapy if indicated. It is noteworthy that the USA and some countries in Europe recommend population-based screening of adults for HCV and risk-based screening for HBV.[15,16]
The Nigerian Society for Gastroenterology and Hepatology in the hepatitis B and C treatment guideline published in 2015[7] also recommends population-based screening of all adults for HBV, with opportunities to screen at any visit to a healthcare facility, antenatal care, preschool and pre-employment, among others. Risk-based screening is recommended for HCV, and the suggested target population includes persons who abuse injection drugs, healthcare workers, persons with sexually transmitted infections, especially HIV, and children born to HCV-positive mothers.[7] The Federal Ministry of Health had earlier adopted population-based voluntary counselling and testing for HIV, which has greatly contributed to reduction of the national prevalence of HIV from 8% in 2006 to 3.5% in 2013. [17] Most prevalence data on HBV and HCV in Nigeria are hospital based, among cohorts of either patients with liver disease or blood donors.[11,18] The same applies to data on HIV prevalence in Nigeria, which have largely been derived from sentinel studies among pregnant women and other people presenting for care in health facilities.[19,20] It is vital to acquire population-based data on the prevalence of these blood-borne viruses to inform optimal national control strategies.
Objective
To determine the population-based prevalence of hepatitis B and C viruses and HIV and risk factors for infection with these viruses in CRS, Nigeria.
Methods
Study area
The study was conducted in CRS, located in the coastal region of Nigeria. The state occupies 20 156 km2 and shares a boundary with the Republic of Cameroon. According to the 2006 national census, CRS had a total population of 2 892 988, with 1 471 967 males and 1 421 021 females.[6] CRS is divided into three senatorial districts (southern, central and northern) and 18 local government areas (LGAs), six per senatorial district. They are Abi, Akamkpa, Akpabuyo, Bakassi, Bekwarra, Biase, Boki, Calabar Municipal, Calabar South, Etung, Ikom, Obanliku, Obubra, Obudu, Odukpani, Ogoja, Yakuur and Yala.
Study design
The study was a cross-sectional analytical study conducted between March 2015 and August 2016.
Study population
The study population consisted of residents of CRS aged >18 years. Each respondent provided written informed consent. Individuals aged <18 years and those who refused to give consent were excluded from the study.
Sample size calculation
The minimum sample size for this study was 1 620, which was calculated using the formula:
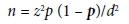
where z = confidence interval (CI) of 95%, p = prevalence rate of hepatitis B infection taken as 12%,[18] and d = the desired precision, which was 5%.
Sampling technique
A multistage sampling method was used to recruit participants.
• Stage 1. In each senatorial district, three LGAs were randomly selected by balloting. The selected LGAs were Calabar south, Akpabuyo, Akamkpa, Abi, Yakurr, Ikom, Obudu, Ogoja and Yala.
• Stage 2. In each selected LGA, three wards were further selected randomly by balloting.
• Stage 3. A total of 60 participants were recruited from each ward.
Advocacy visits to community leaders were carried out and community mobilisation was conducted to sensitise the residents prior to the screening exercise.
Data collection
A structured questionnaire, pretested for reliability and validity and including demographic data (age, sex and family size), past/present symptoms of liver disease, and exposure to risk factors associated with acquisition of the hepatitis/HIV viruses was administered to each participant.
Laboratory investigations
Three millilitres of venous blood was collected from each participant into a clean plain bottle, allowed to clot and then centrifuged at 3 000 rpm for 10 minutes. The supernatant serum was harvested and pipetted into cryotubes, which were stored at -20oC till the time of batch analysis. Each sample was tested for HBV, HCV and HIV using an enzyme-linked immunosorbent assay (ELISA) technique with assay kits manufactured by DRG International Inc. (USA). The hepatitis B surface antigen (HBsAg) ELISA kit utilises horseradish peroxidase (HRP)-conjugated anti-human IgG and multiple recombinant HBsAg antigens to detect HBsAg in serum samples. Test results were interpreted as a ratio of the absorbance of the sample (As) and the absorbance of the negative control (An), an As/An ratio of <2.10 indicating a negative result and a ratio of >2.10 a positive result. Assay sensitivity and specificity were both >97.5%.
Sera were also tested for anti-HCV IgG antibodies using a sandwich ELISA technique. The HCV ELISA test kit utilises HRP-conjugated anti-human IgG and multiple recombinant HCV antigens to detect anti-HCV IgG antibodies in serum samples. Test results were interpreted as the ratio of the absorbance of samples and cut-off absorbance (COV), determined as the mean of the absorbances of two negative control sera + 0.15. A ratio of <1.0 indicates a negative result (absence of anti-HCV IgG antibodies) while a ratio of >1.0 indicates a positive result (presence of detectable anti-HCV IgG antibodies in serum samples).
HIV 1 and 2 antibodies were tested for using a sandwich ELISA technique. The test kit utilises HRP-conjugated HIV antigen and recombinant HIV antigen coated on micro-wells to detect anti-HIV antibodies present in serum samples. Test results were interpreted based on the calculated COV determined as the average of absorbances of two negative control sera + 0.1. Samples with absorbances >COV absorbance were reported as positive, while those with absorbance <COV absorbance were reported as negative. Assay sensitivity, specificity and imprecision were >97.5%, >97.5%, and <15.0%, respectively.
Statistical analyses
Stata version 14 (StataCorp, USA) was used for the data analyses. The Kolmogrov-Smirnov test was used to test the nature of distribution of continuous variables. Median values were calculated for skewed continuous variables, while categorical variables were presented in percentages. Bivariate analyses (χ2 and Fisher's exact tests) were used to compare differences in sociodemographic characteristics and other categorical attributes. Risk factors that were significantly associated with HBV, HCV and HIV infections were analysed by forward selection and used to model the multivariate (adjusted) binary logistic regression analysis at a 5% level of significance. Risk factors that were not significantly associated (CIs including the null value of 1) with HBV, HCV and HIV infections were excluded in the final adjusted model. The multiple imputations by chained equations approach for categorical variables was used to impute for risk factors with missing observations.
Ethics approval
Ethics approval was obtained from the CRS Health Research Ethics Committee (ref. no. RP/REC/2015/281).
Results
A total of 1 498 subjects were recruited from the three senatorial districts in CRS. The southern, central and northern districts contributed 536 (35.8%), 445 (29.7%) and 517 (34.5%) of the study population, respectively. The median age of the study population was 39 years (interquartile range (IQR) 27 - 50), and was higher for individuals from the central district than for those from the southern and northern districts (40 years v. 35 years; p=0.001). There were more females than males (average ratio 1.7:1), the variation in gender ratio being more pronounced in the central district (2.1:1) than in the southern and northern districts (1.9:1 and 1.3:1). The majority of the participants were married, had a formal education (usually a tertiary education), and came from households with >5 members. Further characteristics of the study population are summarised in Table 1.
Characteristics of HBsAg-positive subjects
A total of 1 365 samples were assayed for HBsAg. The prevalence of HBsAg was 8.8% (n=120). HBsAg-positive subjects were more likely to be <48 years of age than older (72.5%) and to be from the northern district than the other districts (52.5%). HBsAg positivity tended to decrease with increasing age, and the difference was statistically significant (p=0.011). There was no significant association between sex and HBsAg status, although more males than females were affected (9.7% v. 8.3%; p=0.08) (Table 2).
In the multivariate regression analysis, only two risk factors in the final model, communal use of a toothbrush and residence in the northern district, were significantly associated with HBsAg positivity (odds ratio (OR) 2.46, 95% CI 1.45 - 4.18 and OR 2.16, 95% CI 1.31 -3.55, respectively) (Table 3).
Characteristics of anti-HCV positive subjects
A total of 1 364 samples were assayed for anti-HCV. The anti-HCV prevalence overall was 10.0% (n=137), the prevalence being lowest in the southern district (2.8%) and highest in the northern district (18.2%). This difference was statistically significant (p<0.001). The age group 20 - 37 years (23.4%) was most frequently affected. More males than females were anti-HCV-positive (10.7% v. 9.4%) (Table 2). In stepwise logistic regression, older age (>57 years) and senatorial district showed a significant association with anti-HCV positivity in the final model (OR 2.24, 95% CI 1.47 - 3.41 and OR 2.84, 95% CI 1.50 - 5.39, respectively) (Table 4).
Characteristics of HIV-positive subjects
A total of 1 350 samples were assayed for HIV. The prevalence of HIV was 12.9% (n=174). The highest prevalence was recorded in the southern district (23.5%), followed by the northern and central districts (8.1% and 6.5%). The prevalence of HIV infection decreased with increasing age, especially in the southern district, with the 18 -27-year age group being worst affected. The association between age and HIV positivity was statistically significant (p=0.001). Although more females than males had a positive HIV result (14.0% v. 11.8%), the difference was not statistically significant (p=0.09) (Table 2). In multivariate regression analysis, only senatorial district was associated with HIV positivity in the final model (OR 3.97, 95% CI 2.43 - 6.47) (Table 5).
The prevalences of HBV/HCV, HIV/HBV, HIV/HCV and HIV/HBV/HCV co-infection were 1.3%, 1.3%, 1.5% and 0.4%, respectively. The rate of HBV/HCV co-infection was highest in the northern district (2.6%), that of HIV/HBV co-infection was highest in the southern district (1.7%), and that of HIV/HBV/HCV co-infection was highest in the central district (0.8%). There was no significant variation between co-infection and senatorial districts except for HBC/HCV co-infection (p=0.009). Only 19.5% of the studied population reported having received vaccination for HBV.
Discussion
HBV, HCV and HIV infections are major public health concerns in CRS and Nigeria as a whole, not only because Nigeria is a hyper-endemic region, but also because individuals infected with these viruses may remain asymptomatic for many years and serve as reservoir of infection during this period. The need to establish the true burden of disease in our various communities therefore cannot be over-emphasised. The 8.8% prevalence of HBV in this study is high. It is higher than the 5.6% reported among a cross-section of hospital staff and volunteers in Calabar, the capital of CRS.[11] The difference in prevalence rates could be due to differences in sample size, sample population and sampling technique. However, it is lower than the national average of 13.6%.[18] The higher national prevalence estimate may be modelled on higher rates from other parts of the country. Again, most studies on HBV prevalence in Nigeria are conducted among high-risk groups such as blood donors and patients with liver disease, which may lead to over- estimation of rates.[21,22]
The observed HIV prevalence of 12.9% indicates a higher disease burden in CRS than the 8% recently described by the Cross River State Agency for Control of AIDS in 2013, and is also much higher than the estimated national average of 3.1%.[8,10] It is likely that these data on HIV prevalence in the general population are underestimates, because testing for HIV is usually conducted on persons who present themselves for voluntary counselling and testing. Uptake of HIV testing and counselling is generally reported to be low in Nigeria, with only 23% of males and 29% of females knowing their status.[17] The situation in CRS is unlikely to be very different. Some studies have reported the prevalence of HIV among special risk groups such as female sex workers in Nigeria to be as high as 27.4%.[17] It is possible that as a result of a widespread economic recession and a rise in youth unemployment, formal and informal transactional sexual practices and having multiple sexual partners for financial benefits, reported to be common among young people, may be escalating the prevalence of HIV in CRS.[10] The higher prevalence of HIV in the urban southern senatorial district compared with the rural central and northern districts is similar to findings from other studies that have reported an urban/ rural disparity in HIV prevalence rates.[23]
The HCV prevalence of 10% in this study is similar to figures reported previously in CRS, with individuals from the northern senatorial district being affected more frequently.[11] Nonetheless, it is higher than the national average of 0.5 - 4%.[7] Unhygienic practices, common in rural communities in the northern part of the state, are thought to be responsible for the high HCV prevalence in that region.[11] Although we observed some association between certain risk factors such as previous blood transfusion, having multiple sexual partners and receiving injections from unqualified persons and positive HCV status, the association was not statistically significant. Obienu et al.[24] have previously reported that risk factors for HCV infection were mostly obscure among Nigerian patients.
Although a higher proportion of our study subjects were female than male, more males than females were positive for HBV and HCV. The reverse was the case for HIV infection. This is in keeping with the national and global trend of unequal gender distribution of infections with these viruses.[4,25] The higher prevalence of infection with the hepatitis viruses among males is thought to be related to the higher clearance rate of these viruses by females compared with males.[26,27] The female preponderance of HIV infection has been attributed to gender inequality, with females being less likely to be able to negotiate safe sex practices.[17]
Individuals aged 18 - 27 years had the highest prevalences of both HBV and HIV infection, while older age was associated with HCV infection. These are similar to findings from other studies.[1,3-4] Mode of transmission, early sexual debut (11 - 15 years) and increased sexual activity among 18 - 27-year-olds are postulated as reasons for this observation. The prevalences of HIV/HBV and HIV/HCV co-infection were low in our study compared with other studies.[28] This may be due to differences in sample size and sample population.
Study limitations
Our study had some limitations. Some of the participants who gave consent refused to give a blood sample. This resulted in disparity between the total study population and the reported population whose blood samples were assayed. The micro-well reader developed a technical problem in the course of HIV analysis, resulting in incorrect coding of a few results.
Recommendations
We recommend that population-based screening programmes be performed in other communities in Nigeria. This will offer the opportunity to identify the majority of individuals with undiagnosed infections, which is necessary to reduce the national burden of disease from complications of these infections such as liver cirrhosis and hepatocellular carcinoma in the future.
Conclusions
Our study showed high prevalences of HBV, HCV and HIV infection in CRS, and infection was unevenly distributed between the urban and rural districts. The burden of HCV in Nigeria may be higher than previously documented, especially in rural communities. Population-based screening guidelines are required to provide vital data that will inform optimal national control strategies.
REFERENCES
1. World Health Organization. HIV and hepatitis co-infection. http://who.int/hiv/topics/hepatitis/en (accessed 5 September 2016). [ Links ]
2. Kourtis AP, Bulterys M, Hu DJ, et al. HIV-HBV co-infection - a global challenge. N Engl J Med 2012;366(19):1749-1752. https://doi.org/10.1056/NEJMp1201796 [ Links ]
3. Platt L, Easterbrook P, Gower E, et al Prevalence and burden of HCV co-infection in people living with HIV: A global systematic review and meta-analysis. Lancet Infect Dis 2016;16(7):797-808. https://doi.org/10.1016/s1473-3099(15)00485-5 [ Links ]
4. Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat 2004;11(2):97-107. https://doi.org/10.1046/j.1365-2893.2003.00487.x [ Links ]
5. CDC Centers for Disease Control and Prevention. HIV/AIDS. http://cdc.gov/hiv (accessed 7 September 2016). [ Links ]
6. National Population Commission, Nigeria. http://www.population.gov.ng/ (accessed 4 March 2015). [ Links ]
7. Malu AO, Borodo MM, Ndububa DA, et al. Hepatitis B and C treatment guidelines for Nigeria, 2015. Niger J Gastroenterol Hepatol 2015;7(2):63-75. [ Links ]
8. Global AIDS update, 2016. http://www.unaids.org/en/resources/documents/2016/ (accessed 7 September 2016). [ Links ]
9. Arodiwe EB, Nwokediuko SC, Ike SO. Medical causes of death in a teaching hospital in South-Eastern Nigeria: A 16 year review. Niger J Clin Pract 2014;17(6):711-716. https://doi.org/10.4103/1119-3077.144383 [ Links ]
10. Cross River State Social and Behaviour Change and Communication Strategy. 2013. http://crsaca.gov.ng/ (accessed 14 September 2016). [ Links ]
11. Kooffreh-Ada M, Okpara H, Oku A, et al. Risk factors for chronic liver disease amongst patients receiving care in a gastroenterological practice in Calabar. IOSR J Med Dent Sci 2015;14(12):6-13. [ Links ]
12. Stockdale AJ, Phillips RO, Beloukas A, et al. Liver fibrosis by transient elastography and virologic outcomes after introduction of tenofovir in lamivudine-experienced adults with HIV and hepatitis B virus coinfection in Ghana. Clin Infect Dis 2015;61(6):883-891. https://doi.org/10.1093/cid/civ421 [ Links ]
13. Knowdley KV, Gordon SC, Reddy KR, et al. Ledispavir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med 2014;370(20):1879-1888. https://doi.org/10.1056/NEJMoa1402355 [ Links ]
14. Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med 2014;370(21):1973-1982. https://doi.org/10.1056/nejmoa1402869 [ Links ]
15. Smith BD, Morgan RL, Beckett GA, et al. Hepatitis C virus testing of persons born during 1945-1965: Recommendations from the Centers for Disease Control and Prevention. Ann Intern Med 2012;157(11):1-38. https://doi.org/10.7326/0003-4819-157-9-201211060-00530 [ Links ]
16. Bechini A, Falla A, Ahmad A, et al. Identification of hepatitis B and C screening and patient management guidelines and availability of training for chronic viral hepatitis among health professionals in six European countries: Results of a semi-quantitative survey. BMC Infect Dis 2015;15:353. https://doi.org/10.1186/s12879-015-1104-8 [ Links ]
17. Federal Ministry of Health, Nigeria. National HIV & AIDS and Reproductive Health Survey (NARHS Plus II, 2012). November 2013. http://nigeriahealthwatch.com/wp-content/uploads/bsk-pdf-manager/431_2012_National_HIV_&_AIDS_and_Reproductive_Health_Survey_ (NARHS_Plus_II,_2012),_FMOH_Abuja_1172.pdf (accessed 3 September 2016). [ Links ]
18. Musa BM, Bussell S, Borodo MM, et al. Prevalence of hepatitis B virus infection in Nigeria, 2000-2013: A systematic review and meta-analysis. Niger J Clin Pract 2015;18(2):163-172. https://doi.org/10.4103/1119-3077.151035 [ Links ]
19. National AIDS/STDs Control Program (NASCP). National HIV Seroprevalence Sentinel Survey: Process and Findings. Abuja, Nigeria: Federal Ministry of Health, 2008. [ Links ]
20. Okocha EC, Oguejiofor OC, Odenigbo CU, et al. Prevalence of hepatitis B surface antigen seropositivity among HIV-infected and non-infected individuals in Nnewi, Nigeria. Niger Med J 2012;53(4):249-253. https://doi.org/10.4103/0300-1652.107605 [ Links ]
21. Ola SO, Otegbayo JA, Odaibo GN, et al. Serum hepatitis C virus and hepatitis B surface antigenaemia in Nigerian patients with acute icteric hepatitis. West Afr J Med 2002;21(1):215-217. [ Links ]
22. Ado A, Alhassan S, Chonoko UG, et al. Sero-prevalence of hepatitis B surface antigen (HBsAg) among blood donors attending Ahmadu Bello university teaching hospital (ABUTH), Zaria, Nigeria. Bajero J Pure Appl Sci 2010;3(1):20-22. https://doi.org/10.4314/bajopas.v3i1.58557 [ Links ]
23. Bashorun A, Nguku P, Kawu I, et al. A description of HIV prevalence trends in Nigeria from 2001 to 2010: What is the progress, where is the problem? Pan Afr Med J 2014;18(Suppl 1):3. https://doi.org/10.11694/pamj.supp.2014.18.1.4608 [ Links ]
24. Obienu O, Nwokediuko S, Malu A, et al. Risk factors for hepatitis C virus transmission obscure in Nigerian patients. Gastroenterol Res Pract 2011(2011), article ID 939673. https://doi.org/10.1155/2011/939673 [ Links ]
25. Onyekwere CA, Hameed L. Hepatitis B and C virus prevalence and association with demographics: Report of population screening in Nigeria. Trop Doct 2015;45(4):231-235. https://doi.org/10.1177/0049475514560211 [ Links ]
26. London WT, Drew JS. Sex differences in response to hepatitis B infection among patients receiving chronic dialysis treatment. Proc Natl Acad Sci U S A 1977;74(6):2561-2563. [ Links ]
27. Bakr I, Rekacewicz C, El Hosseiny M, et al. Higher clearance of hepatitis C virus infection in females compared with males. Gut 2006;5(8):1183-1187. https://doi.org/10.1136/gut.2005.078147 [ Links ]
28. Balogun TM, Emmanuel S, Ojerinde EF. HIV, hepatitis B and C viruses' coinfection among patients in a Nigerian tertiary hospital. Pan Afr Med J 2012;12:100. [ Links ]
 Correspondence:
Correspondence:
U C Okonkwo
ucsuizes@yahoo.co.uk
Accepted 11 January 2017














