Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.107 no.4 Pretoria Abr. 2017
http://dx.doi.org/10.7196/samj.2017.v107i4.12053
IN PRACTICE
CASE REPORT
Systemic lupus erythematosus: A possible cause of non-alcoholic Wernicke's encephalopathy
M T L NyoI; D MagaziII; M M T M AllyIII
IMB ChB, FCP (SA) Cert Rheum (Phys); Division of Rheumatology, Department of Medicine, Faculty of Health Sciences, Sefako Makgatho Health Sciences University, Pretoria, South Africa
IIMB BCh, MMed (Neuro), FCP (Neuro) (SA); Department of Neurology, Faculty of Health Sciences, Sefako Makgatho Health Sciences University, Pretoria, South Africa
IIIMB BCh, FCP (SA), PhD; Division of Rheumatology, Department of Internal Medicine, Faculty of Health Sciences, University of Pretoria, South Africa
ABSTRACT
We report a young woman with systemic lupus erythematosus (SLE) and an acute cerebellar ataxia. A history of poor appetite and vomiting preceded the inco-ordination. Ataxia in SLE has been well described, but is nevertheless uncommon. The clinical triad of mild confusion, ataxia and ophthalmoplegia also raised the possibility of Wernicke's encephalopathy (WE). The diagnosis of WE was further supported by the magnetic resonance imaging features. Owing to overlapping causal factors, this case illustrates the complexity of diagnosing and managing neuropsychiatric syndromes in a patient with SLE. The limited published literature on SLE-related cerebellar syndromes adds to the challenge. Gastrointestinal manifestations of SLE are described as being common in SLE, with nausea and vomiting occurring in >50% of cases in some series. Poor eating habits and vomiting are well-described causes of non-alcoholic WE. This is the first description of gastrointestinal SLE as a possible cause of WE.
Systemic lupus erythematosus (SLE) is a multisystemic autoimmune disorder, with neurological manifestations being common. Attribution of neurological features to SLE is often a challenging process that requires a thorough clinical evaluation, relevant laboratory investigations and imaging studies. Acute cerebellar ataxia has been described in SLE, although little has been published in this regard. Wernicke's encephalopathy (WE) is a neurological complication of thiamine deficiency. Poor eating habits and vomiting are well-described causes of non-alcoholic WE. Gastrointestinal manifestations commonly occur, with nausea and vomiting reported in >50% of cases in some series. It is important that WE is considered in patients with SLE and ataxia, as it is treatable and carries a significant morbidity and mortality without appropriate treatment.
Case report
A 24-year-old black female patient was referred to the rheumatology clinic at Dr George Mukhari Academic Hospital, a tertiary hospital in Pretoria, South Africa, with suspected SLE. Dermatologists had been treating her for a biopsy-proven bullous lupus for a few months. The initial consultation at the rheumatology clinic confirmed the diagnosis of SLE, based on the presence of acute cutaneous lupus, non-scarring generalised alopecia, haemolytic anaemia, positive antinuclear antibody, positive anti-Smith antibody, a weakly positive lupus anticoagulant test, and a low level of serum complement 3 (C3), unequivocally fulfilling the Systemic Lupus International Collaborating Clinics classification criteria for SLE.[1] Although her bullous lesions had healed, she had an active interarticular rash on her fingers and haemolytic anaemia, with a haemoglobin of 6.4 g/dL, confirming the presence of active disease. She received 2 U of blood and was treated with oral prednisone and oral azathioprine.
Five weeks later, she presented again, complaining of intermittent vomiting and nausea of ~3 weeks' duration. She also reported weight loss and vertigo. A physical examination revealed sinus tachycardia, drowsiness, mild confusion, nystagmus, dysarthria, truncal and limb ataxia and bilateral partial 6th cranial nerve palsies. Her haemoglobin was 11.4 g/dL. All other laboratory tests for detecting active SLE in various organ systems were normal, except for hypoalbuminaemia, with a serum albumin of 26 g/L. Her renal function test was normal and there was no proteinuria. Hypoalbuminaemia seemed to reflect the severity of vomiting and possibly the negative acute-phase response. Typical features of SLE-related protein-losing enteropathy, such as diarrhoea, profound pitting oedema and effusions in serous cavities, were absent. Barium studies and gastroscopy did not show any abnormalities to explain the reasons for her vomiting, suggesting that SLE itself was the likely cause. SLE activity was thought to be the cause of sinus tachycardia, as other causes such as thyrotoxicosis, lung diseases, pulmonary embolism and myocarditis were excluded. With regard to her neurological symptoms and signs, the possibility of SLE-related cerebellar syndrome was initially considered in view of active SLE.
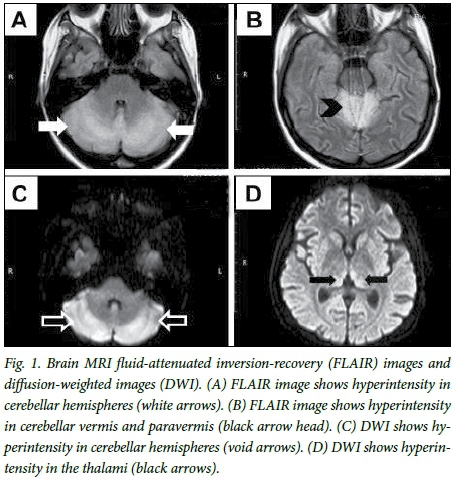
A computed tomography scan of the brain revealed cerebral atrophy, which is not an infrequent finding in patients with SLE. Subsequently, a lumbar puncture and analysis of the cerebrospinal fluid were normal, except for slightly elevated protein at 0.72 g/L; infection-related meningitis was excluded. Magnetic resonance imaging (MRI) scanning revealed signal hyperintensity in the thalami, mid-line cerebellum and cerebellar hemispheres (Fig. 1). The clinical triad of mild confusion, ataxia and ophthalmoplegia in the presence of suggestive MRI findings led to the diagnosis of non-alcoholic WE. It was, however, considered an atypical presentation, as the patient had a pancerebellar syndrome involving the trunk and the limbs, rather than the typical midline cerebellar syndrome of WE that involves mainly the trunk. Dysarthria is also not a common clinical feature of WE.[2]
Case management
The patient was initially treated with intravenous fluid and empiric intravenous antibiotics. In view of the likelihood of gastrointestinal SLE, the low exclusion probability of SLE-related cerebellar syndrome, and low probability of sepsis, she was initially given three doses of intravenous methylprednisolone over 3 days at 750 mg/dose, followed by oral prednisone. Vomiting stopped after a few days. The patient was then started on high-dose intravenous thiamine for 2 weeks, followed by oral thiamine. Two weeks after the initiation of high-dose thiamine treatment, she was found to have some residual ataxia and confusion. Every 2 weeks six intravenous cyclophosphamide pulses at 500 mg/dose were added to her treatment.
Outcome and follow-up
At the completion of all six pulses of cyclophosphamide, the patient was assessed as being in remission from SLE, with no residual neurological features, except for mild forgetfulness reported by the patient. At her most recent follow-up visit about 1 year later, she remained in remission, with full neurological recovery.
Discussion
A major challenge in the diagnosis and management of the index patient was the decision to attribute her cerebellar ataxia to the SLE as opposed to an SLE-unrelated cause. This is not an uncommon challenge in the setting of neurological manifestations of SLE owing to an overlap in presentation. The main alternative diagnostic consideration in this case was non-alcoholic WE.
The process of attributing a neuropsychiatric event to SLE usually involves: (i) exclusion of secondary causes; (if) assessment of the type of event and its timing in relation to the onset of SLE; (iii) assessment of risk factors for SLE-related events v. SLE-unrelated events; (iv) special investigations, often including neuroimaging and analysis of cere-brospinal fluid; and (v) assessment of clinical response to treatment.[3]
Secondary causes such as drugs, a paraneoplastic phenomenon and metabolic abnormalities causing cerebellar ataxia were excluded and no source of infection was identified.
Regarding the type of event, it has been shown that major occurrences, such as an acute confusion state, refractory seizures, myelopathy, optic neuritis, acute psychosis, polyneuropathy and stroke, are more likely to be attributable to SLE than minor events, such as mild depression, anxiety, headaches, mild cognitive dysfunction and polyneuropathy without electrophysiological evidence.[4] However, there is a scarcity of information about SLE-related cerebellar ataxia in the published literature. The American College of Rheumatology described 19 neuropsychiatric syndromes in 1999[5] - one of these is movement disorders. Cerebellar ataxia together with chorea and hemiballismus are categorised as movement disorders. In 1988, Singh et al.[6] reported three women with SLE and cerebellar ataxia, who responded favourably to treatment with moderate doses of prednisone. In 1996, Manto et al.[7]reported a 27-year-old woman with SLE, who presented with a subacute pancerebellar syndrome. Her brain MRI scan showed cerebellar atrophy and the cerebellar ataxia improved markedly following corticosteroid administration. In 2000, Yaginuma et al.[8] described a 28-year-old woman with active SLE involving digestive tract, skin and kidneys, who developed acute cerebellar ataxia, a paresis of the right inferior rectus muscle, left abducens paralysis and left facial palsy, which seemed to be consistent with a brainstem lesion visible on MRI. This lesion disappeared within 9 days of corticosteroid treatment. In 2008, Appenzeller et al.[9] reported 3 women with SLE and acute cerebellar ataxia, who were successfully treated with intravenous corticosteroids and an immunosuppressant. Most recently, in 2014, Ghosh et al.[10]described a 22-year-old girl with active SLE, who presented with lupus nephropathy and subacute pan-cerebellar syndrome. Her brain MRI scan showed cerebellar atrophy. Treatment of lupus nephropathy with oral corticosteroids and myco-phenolate mofetil prevented further deterioration of her cerebellar syndrome.
Regarding the timing of the event, an SLE-related neuropsychiatric event tends to occur at the onset of SLE or within the first 2 years after the diagnosis.[3] Our patient seemed to fall into the latter category.
Major risk factors for SLE-related events include generalised SLE activity or damage, history of previous or concurrent major SLE-related neuropsychiatric events and presence of antiphospholipid antibodies.[3] Our patient had a weakly positive lupus anticoagulant test. The following features may have suggested active SLE in this patient: sinus tachycardia, hypoalbuninaemia, low serum C3 level and possible gastrointestinal SLE.
MRI findings of our patient were not typical for an SLE-related neuropsychiatric event. There was no evidence of ischaemia, as would have resulted from a vasculitis or vasculopathy. The involvement of the vermis, the paravermis together with the medial aspects of both thalami, has been described by some researchers as specific for nonalcoholic WE.[11,12] The involvement of the cerebellar hemispheres, albeit atypical, has been described in WE.[13]
Gastrointestinal manifestations are described as being common in SLE, with nausea and vomiting occurring in >50% of cases in some series.[14] In our patient, the acute cerebellar syndrome, together with the MRI features, was suggestive of WE. Poor eating habits and vomiting are well-described causes of non-alcoholic WE.[15]
WE is a neurological complication of thiamine (vitamin B1) defi-ciency.[2] It is an acute syndrome requiring emergent treatment to prevent death and neurological morbidity. Women appear to be more susceptible to the condition than men. Although chronic alcoholism accounts for most cases of WE, it can be associated with other conditions such as anorexia nervosa, dieting, starvation, gastrointestinal surgery or hyperemesis gravidarum. The classic triad of encepha-lopathy, oculomotor dysfunction/ophthalmoplegia and gait ataxia may not be present in all patients; hence, a high index of suspicion is necessary to recognise and treat the condition early. Ataxia of the legs or arms and dysarthria or scanning speech are thought to be uncommon, although some literature suggests that the lower limbs are typically involved. Diagnosis is primarily clinical because the specific diagnostic laboratory tests (e.g. red blood cell thiamine) are often not readily available. A brain MRI scan typically shows signal hyperin-tensities in the periaqueductal area, medial thalamus, dorsal medulla, tectal plate and mamillary bodies. Treatment of WE is essentially with high-dose intravenous thiamine, followed by oral thiamine. Response to thiamine replacement is usually very good and improvement in ocular signs and confusion can be seen within hours to days, while gait ataxia may start improving only in the second week.
Conclusion
Owing to overlapping causal factors, this case illustrates the complexity of diagnosing and managing neuropsychiatric syndromes in patients with SLE. The limited published literature on SLE-related cerebellar syndrome further contributes to the diagnostic dilemma. Ataxia is not common in SLE; when it occurs, WE is an important consideration because it is treatable and carries a significant mortality rate, should it be ignored.[16] In our case, given the clinical presentation, which was suggestive of WE owing to gastrointestinal SLE, we instituted treatment for both conditions, with a satisfactory outcome. This is the first description of gastrointestinal SLE as a possible cause of non-alcoholic WE.
Teaching points
• Diagnosing and managing neuropsychiatric syndromes in patients with SLE is a challenging process, requiring a multidisciplinary approach.
• Poor eating habits and vomiting are well-described causes of non alcoholic WE.
• Gastrointestinal manifestations, such as vomiting, are common in SLE.
• Gastrointestinal SLE is a possible cause of non-alcoholic WE.
• WE is an important consideration in a patient with SLE and acute cerebellar ataxia.
REFERENCES
1. Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64(8):2677-2686. https://doi.org/10.1002/art.34473 [ Links ]
2. Yuen T. Wernicke encephalopathy. In: Aminoff MJ, ed. UpToDate 2015. https://uptodate.com/contents/wernicke-encephalopathy?source=search_result&search=wernicke%20encephalopathy&selectedTitle=1~45 (accessed 16 February 2017). [ Links ]
3. Fanouriakis A, Boumpas DT, Bertsias GK. Pathogenesis and treatment of CNS lupus. Curr Opin Rheumatol 2013;25(5):577-583. https://doi.org/10.1097/BOR.0b013e328363eaf1 [ Links ]
4. Ainiala H, Hietaharju A, Loukkola J, et al. Validity of the new American College of Rheumatology criteria for neuropsychiatric lupus syndromes: A population-based evaluation. Arthritis Rheum 2001;45(5):419-423. https://doi.org/10.1002/1529-0131(200110)45:5<419::AID-ART360>3.0.CO;2-X [ Links ]
5. American College of Rheumatology. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum 1999;42(4):599-608. https://doi.org/10.1002/1529-0131(199904)42:4<599::AID-ANR2>3.0.CO;2-F [ Links ]
6. Singh RR, Prasad K, Kumar A, et al. Cerebellar ataxia in systemic lupus erythematosus: Three case reports. Ann Rheum Dis 1988;47(11):954-956. https://doi.org/10.1136/ard.47.11.954 [ Links ]
7. Manto MU, Rondeaux P, Jacquy J, et al. Subacute pancerebellar syndrome associated with systemic lupus erythematosus. Clin Neurol Neurosurg 1996;98(2):157-160. https://doi.org/10.1016/0303-8467(96)00013-3 [ Links ]
8. Yaginuma M, Suenaga M, Shiono Y, et al. Acute cerebellar ataxia of a patient with SLE. Clin Neurol Neurosurg 2000;102(1):37-39. https://doi.org/10.1016/S0303-8467(99)00078-5 [ Links ]
9. Appenzeller S, Cendes F, Costallat LT. Cerebellar ataxia in systemic lupus erythematosus. Lupus 2008;17(12):1122-1126. https://doi.org/10.1177/0961203308093071 [ Links ]
10. Ghosh K, Chatterjee A, Ghosh S, et al. Cerebellar ataxia in a young patient: A rare path to lupus. J Neurosci Rural Pract 2014;5(Suppl 1):S75-S76. https://doi.org/10.4103/0976-3147.145212 [ Links ]
11. Fei GQ, Zhong C, Jin L, et al. Clinical characteristics and MRI features of nonalcoholic Wenicke encephalopathy. Am J Neuroradiol 2008;29(1):164 -169. https://doi.org/10.3174/ajnr.A0827 [ Links ]
12. Roh JH, Kim JH, Koo Y, et al. Teaching neuroimage: Diverse MRI signal intensities with Wernicke encephalopathy. Neurology 2008;70(15):e48. https://doi.org/10.1212/01.wnl.0000308951.21103.49 [ Links ]
13. Kim JE, Kim TH, Yu IK, et al. Diffusion-weighted MRI in recurrent Wernicke's encephalopathy: A remarkble cerebellar lesion. J Clin Neurol 2006;2(2):141-145. https://doi.org/10.3988/jcn.2006.2.2.141 [ Links ]
14. Xu D, Yang H, Lai CC, et al. Clinical analysis of systemic lupus erythematosus with gastrointestinal manifestations. Lupus 2010;19(7):866-869. https://doi.org/10.1177/0961203310365883 [ Links ]
15. Galvin R, Brathen G, Ivashynka A, et al. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol 2010;17(12):1408-1448. https://doi.org/10.1111/j.1468-1331.2010.03153.x [ Links ]
16. Pearce J. Wernicke-Korsakoff encephalopathy. Eur Neurol 2008;59(1-2):101-104. https://doi.org/10.1159/000109580 [ Links ]
 Correspondence:
Correspondence:
M T L Nyo
mtlnyo@gmail.com
Accepted 13 January 2017.














