Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.107 no.3 Pretoria Mar. 2017
http://dx.doi.org/10.7196/samj.2017.v107i3.11320
RESEARCH
The impact of a modified World Health Organization surgical safety checklist on maternal outcomes in a South African setting: A stratified cluster-randomised controlled trial
M NaidooI; J MoodleyII; P GathiramIII; B SartoriusIV
IFCFP, PhD;Discipline of Family Medicine, School of Nursing and Public Health, College of Health Sciences, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
IIFCOG, FRCOG, MD; Women's Health and HIV Research Group, School of Clinical Medicine, College of Health Sciences, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
IIIPhD; Discipline of Family Medicine, School of Nursing and Public Health, College of Health Sciences, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
IVPhD; Discipline of Public Health Medicine, School of Nursing and Public Health, College of Health Sciences, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
ABSTRACT
BACKGROUND. In South Africa (SA), the Saving Mothers Reports have shown an alarming increase in deaths during or after caesarean delivery.
OBJECTIVE. To improve maternal surgical safety in KwaZulu-Natal Province, SA, by implementing the modified World Health Organization surgical safety checklist for maternity care (MSSCL) in maternity operating theatres.
METHODS. The study was a stratified cluster-randomised controlled trial conducted from March to November 2013. Study sites were 18 hospitals offering maternal surgical services in the public health sector. Patients requiring maternal surgical intervention at the study sites were included. Pre-intervention surgical outcomes were assessed. Training of healthcare personnel took place over 1 month, after which the MSSCL was implemented. Post-intervention surgical outcomes were assessed and compared with the pre-intervention findings and the control arm. The main outcome measure was the mean incidence rate ratios (IRRs) of adverse incidents associated with surgery.
RESULTS. Significant improvements in the adverse incident rate per 1 000 procedures occurred with combined outcomes (IRR 0.805, 95% confidence interval (CI) 0.706 - 0.917), postoperative sepsis (IRR 0.619, 95% CI 0.451 - 0.849), referral to higher levels of care (IRR 1.409, 95% CI 1.066 - 1.862) and unscheduled return to the operating theatre (IRR 0.719, 95% CI 0.574 - 0.899) in the intervention arm. Subgroup analysis based on the quality of implementation demonstrated greater reductions in maternal mortality in hospitals that were good implementers of the MSSCL.
CONCLUSIONS. Incorporation of the MSSCL into routine surgical practice has now been recommended for all public sector hospitals in SA, and emphasis should be placed on improving the quality of implementation.
There has been considerable debate on the effect of the World Health Organization (WHO) surgical safety checklist (SSCL) on surgical morbidity and mortality.[1] This follows a study that found suboptimal outcomes when the SSCL was introduced in Ontario, Canada.[2] There are varied opinions on the efficacy of the SSCL, with some authors claiming that the sentinel trial commissioned by the WHO had 'inflated attributable benefits'.[3] Haynes et al.[4] showed reductions in the death rate of between 1.5% and 0.8% in their study in eight different hospitals globally. In addition, while many investigators have demonstrated statistically significant reductions in postoperative complications after implementation of the SSCL,[4-7] others have not found the same benefits.[2,8]
Very few studies from low- and middle-income countries (LMICs) have measured the impact of the SSCL on maternal mortality, despite surgical mortality being reported to be five to ten times higher than in high-income countries (HICs), and the rate of deaths due to general anaesthesia being as high as 1 per 150 cases in some parts of sub-Saharan Africa.[9] The experience of two investigators in the present study (MN and JM), who are maternal death assessors in KwaZulu-Natal (KZN) Province, South Africa (SA), is that the SSCL is not being used despite being included in the National Core Standards framework for SA to improve patient safety in hospitals.[10] Surgically related deaths made a significant contribution to the high maternal mortality ratio in SA, with the 6th report of the National Committee for Confidential Enquiry into Maternal Deaths in South Africa,[11] spanning the period 2011 - 2013, placing the risk of a woman dying during or after caesarean delivery (CD) at 185.8 per 100 000 live births, compared with 66.6 per 100 000 live births for women delivering vaginally. Additionally, KZN has the highest CD rate in SA,[11] and the effect of the SSCL on maternal outcomes is not known.
Objective
Given the international aim to reduce maternal mortality to a target of <70 deaths per 100 000 live births, one of the Sustainable Development Goals,[12] the objective of this study was to evaluate the effects of implementing a modified SSCL (MSSCL) on maternal surgical outcomes in KZN.
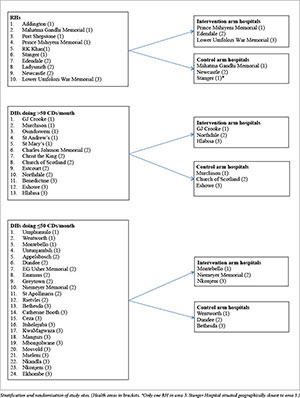
Methods
We conducted a stratified cluster-randomised controlled trial using a two-arm design. Public sector hospitals were stratified into district hospitals (DHs) or regional hospitals (RHs), with the DHs being further classified as large or small based on the number of CDs performed per month. Further geographical stratification occurred based on the three demarcated health areas in the province (Appendix 1). Central and tertiary hospitals were excluded, as they are not found in all the three areas. The sites were then randomised into intervention or control (where no intervention took place) arms, with nine hospitals in each. A computerised system was used to randomly select the hospital clusters for the intervention and control arms. Surgical outcomes were estimated over a period of 9 months - 3 months before intervention (baseline) and 6 months after intervention. Both rural and urban hospitals were included, and they represented 18 (12 DHs and 6 RHs) out of 50 public hospitals in KZN. Information on the number of CDs and the number of deliveries performed at various hospitals in KZN during 2011 was obtained from the District Health Information System. As this was a cluster-randomised control trial, the sample size was worked out by first estimating the average number of CDs done in hospitals that met the eligibility criteria. This was calculated as 85 CDs per month per site. The intra-cluster correlation coefficient (ICC) was obtained for maternal outcomes from a published WHO survey done in 2005.[13] However, the median ICC for maternal outcomes of 0.011 reported was applicable to all maternal outcomes and not just surgical outcomes.[13] Haynes et al.[4] reported inpatient complication rates following surgery of 11% before and 7% after intervention, which translates into a 36% overall reduction. A review investigating complication rates after CD over a 10-year period in The Netherlands[14] reported a complication rate of 50.5%, which was adjusted when all the minor postoperative complications were removed. This gave an incident rate of 512 events among 2 647 CDs, which equated to a complication rate of 19.3%, slightly higher than the WHO estimate of 3 - 16%.
When the sample size for this study was calculated, the following were taken into account:[15] (i) the sample size was calculated for binary outcomes that were used in the study; (ii) the number of clusters in each group was calculated with an 80% power; (iii) a 5% significance level was used; (iv) the average cluster size was 85 patients per month over a 6-month period (n=510 patients); (v) the ICC used was 0.011; (vi) the complication rate in the control group was estimated at 19.34%; and (vii) the complication rate expected for the intervention group would be reduced by 36.36%, giving a rate of 12.31%.
Sample size (regional and district hospitals) as well as the number of hospitals in each arm (control and intervention arms) was calculated using Stata: Release 13 Statistical Software (StataCorp, USA). The sample size estimate for binary outcomes using an effect size based on proportions in this experimental study was calculated as 422 per group. This was multiplied by the design effect used in cluster-randomised trials, i.e. 1 + (n - 1) p, where n is the sample size per cluster (510) and p is the ICC.[15] This gave a sample size of 3 785. Divided by the average sample size of 510, this gave a figure of six clusters for both the intervention and control groups. However, because of the variability between district hospitals in terms of size and geographical location, it was decided to stratify them further based on geographical location and hospital size in relation to maternal surgical workload. In total, nine clusters per arm of the study (18 clusters) were selected.
The MSSCL
The intervention on the use of the MSSCL consisted of training by the principal investigator (MN) of doctors and nurses working in maternity operating theatres during May 2013. The MSSCL used was the SSCL adapted by the provincial health department of the Western Cape Province of SA and further modified by us (we deleted an item on scalp vein electrodes). The MSSCL consists of three sections, the sign-in phase, the time-out phase and the sign-out phase.
Before induction of anaesthesia (sign in)
During this phase the identity of the woman, the procedure and consent is confirmed. The anaesthetist and paediatrician/midwife confirm that the anaesthetic and neonatal safety checks are complete with no problems. A pulse oximeter is confirmed to be on the patient and in working order. The surgeon and anaesthetist then confirm that the patient has no allergies and does not have a difficult airway, and that there is no risk of aspiration or excessive blood loss. This phase should ideally take 30 - 60 seconds.
Before the skin incision (time out)
This occurs after induction of the anaesthetic. During this phase all members would have introduced themselves. The patient identity and procedure are again confirmed. The surgeon reviews whether additional procedures are planned and whether there are concerns about the placental site. The anaesthetist also states whether he/she has any concerns about the patient, and the nursing team confirms sterility of the instruments and that there are no equipment concerns. The team confirms that prophylactic antibiotic/s, antacids and, if applicable, antiretrovirals have been administered to the patient. This process should also take about 30 - 60 seconds.
Before the patient leaves the operating room (sign out)
This occurs at the end of the operation. The nurse confirms that the procedure has been recorded and that the instrument, swab and needle count is correct. Specimens are confirmed to be appropriately labelled. Blood loss is confirmed to have been recorded. If there were any equipment concerns, these problems have to be addressed before the next procedure. The baby/babies are correctly identified. The surgeon, anaesthetist and recovery room nurse review the key concerns for recovery and decide whether the patient needs further management. This process should take 30 - 60 seconds.
Data collection and statistical analysis
Institutional monthly data collection sheets contained count numbers of the various maternal operations performed, the total number of births, and intraoperative complications such as airway problems, hypotension, haemorrhage, surgical complications and deaths. Postoperative complications consisted of monthly count data of post-partum haemorrhage (PPH), shock, sepsis, deep-vein thrombosis (DVT) or pulmonary embolus, referral to a higher level of care (an RH in the case of DHs or an intensive care unit (ICU) for RHs), unscheduled return to the operating theatre, postoperative deaths and other postoperative complications not listed on the data sheet. Patients who had operations at other hospitals and were subsequently referred to an RH were excluded from the count data of that RH. Data collection sheets were left with theatre staff, labour ward staff and staff from the postoperative wards for them to complete, and these were collected by the principal investigator. In addition, data were extracted from the following sources: (i) operative theatre register; (ii) adverse incident books/files; (iii) operative notes in theatre; (iv) labour ward register; (v) admission books in the postnatal wards; and ( vi) high-care and ICU admission books. In addition, data relating to sepsis after surgery were obtained from the infection control manager, and data on surgically related deaths were provided by the labour ward operational manager and the obstetrician/medical officer in charge. All the data were finally collated onto one monthly surgical outcome data collection tool. Data collection involved visiting the hospitals monthly for the first 3 months and then every 2 months for the next 6 months.
Data were verified by the principal investigator before being entered as a valid event in the data collection tool. Compliance with the MSSCL was evaluated by direct observation of an operative practice and by focus group discussions at the end of the study.
Data were processed and analysed using Stata: Release 13 Statistical Software. Complication rates were expressed as proportions of the operative procedures before and after intervention. An incident rate per 1 000 procedures was used for comparison of events at individual hospitals before and after intervention. A random-effects Poisson modelling approach was used to compare counts in hospitals in the intervention and control arms of the study. A within-facility random effect was incorporated to account for correlated (repeated) monthly measurements in each hospital to correctly estimate the significance when comparing pre- and post-intervention periods. This statistical model took into consideration monthly baseline outcomes (March -May 2013) and monthly intervention outcomes (June - November 2013) using count data, and factors the heterogeneity between the individual hospitals. Furthermore, an adjustment for multiple testing (given multiple outcomes) was performed to reduce the likelihood of a type I error (increased false-positive finding rate) using the Simes correction. Subgroup analysis involved comparing hospitals that implemented the MSSCL (good or poor implementation) with hospitals in the control arm. Observations on the quality of implementation were done by the principal investigator during visits to the institution when data were collected, and the quality of implementation was confirmed when focus group discussions were held at the hospitals that implemented the MSSCL. A very small proportion of operations were observed directly.
Ethical considerations
Ethical approval was obtained from the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (UKZN) (ref. no. BE216/12) and institutional permission was provided by the KZN provincial Department of Health (ref. no. hrkm 146/12). The trial was registered with Pan African Clinical Trails Registry (PACTR201501000981262) retrospectively.
Results
Fig. 1 summarises the trial outline using the Consolidated Standards of Reporting Trials (CONSORT) flow diagram for cluster-randomised controlled trials.
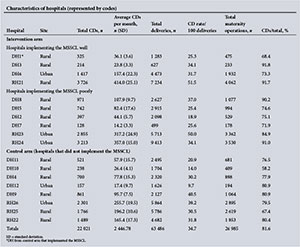
Ten hospitals implemented the MSSCL, with one small district hospital (DH1) from the control arm also implementing it despite not having any formal training on its use. Four hospitals implemented the checklist well. Characteristics of the hospitals in terms of the volume of obstetrics, site and obstetric surgical load for the 9 months of data collection in 2013 from each arm of the study are presented as supporting information in Appendix 2. The maternal surgery consisted of CDs, laparotomies for ectopic pregnancies, uterine evacuations, manual removal of placentas and unplanned returns to theatre.
CDs comprised 81.6% of all the operations reviewed (74.5% of all maternal surgery performed at the DHs and 83.8% at the RHs). The CD rate was 26.9% at the DHs and 39.7% at the RHs. There were 41 maternal deaths, the main causes of death being PPH (n=12, 29.3%), advanced HIV infection (n=7, 17.1%), pregnancy-related infection (n=5, 12.2%) and intraoperative haemorrhage (n=3, 7.3%). The adverse incident rates per 1 000 procedures for intervention and control hospitals, representing combined number of adverse incidents occurring intraoperatively and postoperatively (PPH, shock, sepsis, unscheduled return to the operating theatre, DVT, pulmonary embolus and deaths), are shown in Fig. 2.
The numbers of events in the different arms, as well as the event rates per 1 000 procedures, are set out in Table 1.
Referral to a higher level of care (ICU, RH or tertiary hospital) was not regarded as an adverse event, as it indicated awareness on the part of the healthcare worker (HCW) that the patient required closer monitoring and more intensive postoperative care. Table 2 reflects the change in combined and individual adverse outcomes from baseline in the intervention and control arms.
Combined outcomes (p=0.013), postoperative sepsis (p=0.017) and unscheduled return to the operating theatre (p=0.017) showed significant improvements when the MSSCL was implemented, with referral to higher levels of care (p=0.052) showing marginal significance. Incidence rate ratios (IRRs) for the intervention arm v. the control arm from baseline to post intervention as per three sensitivity analysis scenarios ((i) as per protocol; (if) one hospital in the control arm that incorrectly implemented intervention allocated to the intervention arm; and (iii) one hospital in the control arm that incorrectly implemented intervention excluded) showed significant improvements in the relative risk in all three scenarios for combined outcomes, unscheduled return to the operating theatre and sepsis (Fig. 3).
Owing to the confounding effect of the intervention having good implementation in some hospitals and poor implementation in others, the subgroup analysis was performed based on the level of implementation at each of the intervention sites, and these were compared with the control arm. Table 3 lists IRRs of hospitals with good and poor implementation of the MSSCL compared with the control arm. For this analysis, DH1 was included in the hospitals that had good implementation.
There were greater reductions in postoperative deaths and total maternal deaths and significant reductions in combined outcomes (p<0.05) in hospitals that were good implementers of the MSSCL compared with the control arm.
In six of the nine hospitals the MSSCL was poorly implemented. Poor implementation ranged from almost no implementation to just ticking off the checklist without engaging with the process. Results from the focus group discussions are summarised below.
Reasons for poor implementation of the MSSCL
• Poor teamwork between doctors and nurses. Nurses complained that doctors refused to fill in the forms. Surgeons only arrived in the operating theatre when the anaesthetic had been administered. Doctors stated that they saw nurses filling in the form and assumed that this was part of the nurses' duties.
• Lack of support from senior HCWs and management. Some of the staff complained that they did not feel supported by senior HCWs, so they were not motivated to implement the MSSCL.
• Personal motivation. It emerged that personal motivation on the part of the HCWs was key to effective implementation. Most hospitals where the MSSCL was poorly implemented demonstrated a very low motivation to improve surgical safety.
Reasons for good implementation of the MSSCL
• Personal motivation. The emergence of checklist champions at four of the sites was key to implementation of the MSSCL at these hospitals. These champions took it upon themselves to personally ensure that the MSSCL was implemented and also monitored its use in the operating theatre. The champions at these four sites were an anaesthetist, a scrub sister, an anaesthetic medical officer and an obstetric medical officer.
• Support from management. The hospitals with good implementation of the MSSCL had greater support from senior clinicians and management than hospitals with poor implementation. In one hospital, the medical manager and the quality assurance manager were actively involved in monitoring and evaluation of the MSSCL.
Discussion
Main findings
The main study findings were significant improvements in combined adverse outcomes, postoperative sepsis and unscheduled return to the operating theatre in the intervention compared with the control arm, with referrals to a higher level of care showing marginal significance. In addition, significant improvements in total and postoperative deaths occurred in hospitals where the MSSCL was well implemented compared with hospitals where the programme was poorly implemented.
Other findings
No significant reductions in surgically related maternal deaths were observed. However, hospitals that implemented the MSSCL well showed a greater reduction in maternal mortality than hospitals with poor implementation, which supports the argument that outcomes may be dependent on the quality of implementation.[16] To date only Haynes et al.[4]have shown significant reductions in mortality, other studies having failed to do so.[2,5-8]
The overall complication rates in our study were lower than those reported by others.[4,5,7] This may be because previous studies were done in tertiary, central or specialised teaching hospitals on high-risk patients, and we specifically excluded such hospitals. Most women in our study were of low preoperative risk. However, the complication rates we observed in hospitals where the MSSCL was being well implemented were slightly higher than those found in a large study by Urbach et al.[2]The significant reduction in overall complication rates in the intervention hospitals in the current study is supported by other studies,[4,7] but this has not been consistently demonstrated.[2,17]
Infection rates in the intervention arm of our study were lower than reported in the literature, with De Vries et al.[5]reporting an infection rate of 4.8% before and 3.3% after intervention, compared with 6.8% before and 6.3% after intervention in the control arm of our study. Similar rates in general surgical patients were reported by Haugen et al.[7]Our low sepsis rate may be due to most patients being of low risk, and the practice of routine preoperative use of prophylactic antibiotics. The marginally significant improvement in sepsis rates in the intervention arm is an important finding in our study, as non-pregnancy-related sepsis and pregnancy-related sepsis feature as two of the top five causes of maternal deaths in SA.[11] Some authors have reported similar findings when using the SSCL,[5,7,17] while others failed to show any significant differences in surgical site infection, sepsis and septic shock.[2,8]
Referrals to a higher level of care increased marginally in the hospitals that implemented the MSSCL, which is considered a positive outcome, as potential problems were identified in the intervention group and action was taken to correct them. The checklist item 'Surgeon, anaesthetist and recovery sister review the key concerns for recovery and management of this patient' serves as a cognitive aid in planning postoperative management. Lubbeke et al.[8] also reported that unplanned admission to the ICU improved significantly. Checklist implementation in previous studies occurred in hospitals where patients had high preoperative risks, requiring ICU monitoring as part of the normal standard of care, and this may account for the reduced reporting of this outcome measure in some studies.[4-7]
Unplanned return to the operating theatre was significantly reduced in the intervention arm, and this was also demonstrated by Urbach et al.[2]However, other studies[8,17] did not show similar benefits. Of note, however, is that the Canadian study[2] and our study had similar patient and health service profiles, as the majority of patients received operative care from HCWs working in district or community hospitals.
In a systematic review of 21 studies, Borchard et al.[18] concluded that the overall compliance rate ranged from 12% to 100%. Key areas that facilitated good implementation were the presence of a checklist champion, effective communication and teamwork, and training of the surgical team. Borchard et al.[18] reported that most studies were done in HICs. Haynes et al.[4] had four sites in LMICs, and six of the safety indicators had compliance rates of 0%, 18.1%, 92.1% and 56.7% at the four hospitals. This translated into mean compliance with all six aspects of the SSCL of 40.5% in LMICs compared with 66% in HICs.[4] We handled non-compliance or poor compliance with use of the MSSCL as a confounder and therefore factored it into the subgroup analysis.
Study strengths and limitations
One of the strengths of the study was the emergence of 'checklist champions'. Limitations were that compliance with the MSSCL was not measured and the quality of implementation was based on its correct use, which was established by convenient observation of surgical practice and focus group discussions. For this study, the principal investigator relied on information sourced from operating theatres, postnatal wards, labour wards, high-care units and ICUs. However, a tendency of staff to under-report adverse events may have led to information bias, because this was not included in the final analysis. In addition, we did not take into account the American Society of Anesthesiologists rating of patients, and this could have had an impact on some of the outcomes observed, especially in regional hospitals.
Conclusions
Significant improvements in complications associated with maternal surgery were observed in this study, and when the MSSCL was implemented correctly there was also an increased reduction in maternal deaths. The relevance of this in an LMIC is substantial, as the maternal mortality ratio is a reflection of the quality of healthcare rendered by the government to its citizens. Recommendations would be to ensure that the SSCL is individually championed in each healthcare institution.
Acknowledgements. The authors acknowledge the assistance of all the HCWs employed by the KZN Department of Health who actively participated in this study.
Funding. The study was supported by the following grants: (i) The UKZN Medical Education Partnership Initiative (MEPI), Enhancing Training, Research and Education (ENTREE) programme (grant no. 5R24TW008863). MEPI is an NIH/PEPFAR-funded grant awarded to UKZN in 2010 that aims to develop or expand and enhance models of medical education in sub-Saharan Africa. (ii) The Discovery Foundation awarded one of the authors (MN) the Academic Fellowship Award in 2013.
Author contributions. MN was the principal investigator in the study, JM and PG were co-investigators and supervisors, and BS was the biostatistician involved. All authors contributed to the final manuscript.
REFERENCES
1. Leape LL. The checklist conundrum. N Engl J Med 2014;370(11):1063-1064. http://dx.doi.org/10.1056/nejme1315851 [ Links ]
2. Urbach DR, Govindarajan A, Saskin R, Wilton AS, Baxter NN. Introduction of surgical safety checklists in Ontario, Canada. N Engl J Med 2014;370(11):1029-1038. http://dx.doi.org/10.1056/NEJMsa1308261 [ Links ]
3. Avidan MS, Evers AS. Surgical safety checklists in Ontario, Canada. N Engl J Med 2014;370(24):2350-2351. http://dx.doi.org/10.3410/f.718308814.793494363 [ Links ]
4. Haynes AB, Weiser TG, Berry WR, et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med 2009;360(5):491-499. http://dx.doi.org/10.1016/j.spinee.2010.04.021 [ Links ]
5. De Vries EN, Prins HA, Crolla RM, et al. Effect of a comprehensive surgical safety system on patient outcomes. N Engl J Med 2010;363(20):1928-1937. http://dx.doi.org/10.1056/NEJMsa0911535 [ Links ]
6. Kwok AC, Funk LM, Baltaga R, et al. Implementation of the World Health Organization surgical safety checklist, including introduction of pulse oximetry, in a resource-limited setting. Ann Surg 2013;257(4):633-639. http://doi.org/10.1097/sla.0b013e3182777fa4 [ Links ]
7. Haugen A, S0fteland E, Almeland S, et al. Effect of the World Health Organization Checklist on Patient Outcomes. Ann Surg 2015;261(5):821-828. http://dx.doi.org/10.1097/sla.0000000000000716 [ Links ]
8. Lubbeke A, Hovaguimian F, Wickboldt N, et al. Effectiveness of the surgical safety checklist in a high standard care environment. Med Care 2013;51(5):425-429. http://dx.doi.org/10.1097/mlr.0b013e31828d1489 [ Links ]
9. Weiser TG, Regenbogen SE, Thompson KD, et al. An estimation of the global volume of surgery: A modelling strategy based on available data. Lancet 2008;372(9633):139-144. http://dx.doi.org/10.1016/s0140-6736(08)60878-8 [ Links ]
10. National Department of Health, South Africa. National Core Standards, a Framework for the Assessment of Health Establishments. Pretoria: NDoH, 2012. [ Links ]
11. Pattinson RC, ed. Saving Mothers 2011-2013: The Sixth Report of the National Committee for Confidential Enquiries into Maternal Deaths in South Africa. Pretoria: Government Printer, 2014. [ Links ]
12. World Health Organization. Maternal mortality 2016. 4 April 2016. http://www.who.int/mediacentre/factsheets/fs348/en/ (accessed 20 June 2016). [ Links ]
13. Taljaard M, Donner A, Villar J, et al. Intracluster correlation coefficients from the 2005 WHO Global Survey on Maternal and Perinatal Health: Implications for implementation research. Paediatr Perinat Epidemiol 2008;22(2):117-125. http://dx.doi.org/10.1111/j.1365-3016.2007.00901.x [ Links ]
14. Van Ham MAPC, van Dongen PWJ, Mulder J. Maternal consequences of caesarean section: A retrospective study of intra-operative and postoperative maternal complications of caesarean section during a 10-year period. Eur J Obstet Gynecol Reprod Biol 1997;74(1):1-6. http://dx.doi.org/10.1016/S0301-2115(97)02725-5 [ Links ]
15. Campbell MK, Thomson S, Ramsay CR, MacLennan GS, Grimshaw JM. Sample size calculator for cluster randomized trials. Comput Biol Med 2004;34(2):113-125. http://dx.doi.org/10.1016/s0010-4825(03)00039-8 [ Links ]
16. Patel J, Ahmed K, Guru KA, et al. An overview of the use and implementation of checklists in surgical specialities - a systematic review. Int J Surg 2014;12(12):1317-1323. http://dx.doi.org/10.1016/j.ijsu.2014.10.031 [ Links ]
17. Rodrigo-Rincon I, Martin-Vizcaino MP, et al. The effects of surgical checklists on morbidity and mortality: A pre- and post-intervention study. Acta Anaesthesiol Scand 2015;59(2):205-214. http://dx.doi.org/10.1111/aas.12443 [ Links ]
18. Borchard A, Schwappach DL, Barbir A, Bezzola P. A systematic review of the effectiveness, compliance, and critical factors for implementation of safety checklists in surgery. Ann Surg 2012;256(6):925-933.http://dx.doi.org/10.1097/sla.0b013e3182682f27 [ Links ]
 Correspondence:
Correspondence:
M Naidoo
naidoom@ukzn.ac.za
Accepted 12 September 2016