Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.107 no.2 Pretoria Fev. 2017
http://dx.doi.org/10.7196/samj.2017.v107i2.11451
RESEARCH
Adenovirus-associated pneumonia in South African children: Presentation, clinical course and outcome
M ZampoliI; Z Mukuddem-SablayII
IMB BCh, FCPaed, Cert Paed Pulm; Department of Paediatrics and Child Health, Red Cross War Memorial Children's Hospital and Faculty of Health Sciences, University of Cape Town, South Africa
IIMB ChB, FCPaed, MMed; Department of Paediatrics and Child Health, Red Cross War Memorial Children's Hospital and Faculty of Health Sciences, University of Cape Town, South Africa
ABSTRACT
BACKGROUND. Viruses have emerged as important aetiological agents of childhood pneumonia.
OBJECTIVE. To investigate the clinical presentation, severity and outcome of adenovirus-associated pneumonia (AVP) in children.
METHODS. A retrospective analysis of AVP cases over 12 months was performed, including demographic, clinical course and outcome (death, persistent lung disease (PLD)) data.
RESULTS. Two hundred and six AVP cases (median age 12 months, interquartile range 6 - 24) were identified; 70 children (34.0%) were malnourished and 14 (6.8%) were HIV-infected. Twenty-nine children (14.1%) developed PLD, which was associated with hypoxia at presentation in 26 cases (89.7%; p=0.01) and necessitated admission to the intensive care unit (ICU) in 18 (62.1%; p<0.01); 18/206 children (8.7%) died. Admission to the ICU (odds ratio (OR) 8.3, 95% confidence interval (CI) 2.3 - 29.0) and a positive blood culture (OR 11.2, 95% CI 2.3 - 54.1) were independent risk factors for mortality.
CONCLUSIONS. Adenovirus is a potential cause of pneumonia and PLD in young children in South Africa. ICU admission and a positive blood culture were associated with poor outcome.
Respiratory tract infections remain a common and significant source of morbidity and mortality in children worldwide. This is of relevance in low- to middle-income countries where pneumonia is an important cause of death in children under 5 years of age.[1] With global access to effective immunisation programmes having resulted in a decline in the incidence of bacterial-associated pneumonia, respiratory viruses have emerged as important aetiological agents in childhood pneumonia. This holds true in high-income countries, where respiratory viruses are the commonest cause of childhood pneumonia.[1,2] Respiratory syncytial virus (RSV) is the leading viral cause of pneumonia in high- and low-income countries.[2-4]
The prevalence of adenovirus by polymerase chain reaction (PCR) testing of nasopharyngeal aspirates in childhood pneumonia cases in South Africa (SA) has been reported to be 19 - 26%.[5,6] However, the pathogenic role of adenovirus in childhood pneumonia is unclear. Some studies report an increased prevalence of adenovirus in pneumonia cases, while others found a similar prevalence in population-matched controls.[6,7] The ability of some adenovirus strains to cause severe and fatal necrotising pneumonia has been described.[8.9] Furthermore, long-term sequelae following adenovirus-associated pneumonia (AVP), including bronchiectasis, bronchiolitis obliterans, unilateral hyperlucent lung and persistent abnormal pulmonary function, have been reported.[8,10-13]
The clinical presentation, complications and outcome of AVP in SA children are unknown. Furthermore, the role of HIV infection and malnutrition in the outcome of AVP is unknown.
Objective
To document the presentation, clinical course and outcome of AVP at a tertiary paediatric referral hospital in SA.
Methods
Study design and patient selection
A retrospective descriptive study was conducted in children 0 -13 years of age admitted with laboratory-confirmed AVP to Red Cross War Memorial Children's Hospital (RCWMCH), a tertiary paediatric hospital in Cape Town, SA. Children admitted from 1 January to 31 December 2011 were included. For the purposes of the study, AVP was defined as clinician-diagnosed pneumonia or lower respiratory tract infection (LRTI; cough, tachypnoea with or without chest indrawing) and positive adenovirus PCR from any respiratory tract samples, which included tracheal aspirates, bronchoalveolar lavage, induced sputum and nose swabs or nasopharyngeal aspirates. Adenovirus subtypes were not identified in routine clinical samples. Children without pneumonia or LRTI documented in the records or laboratory request form details were excluded. The National Health Laboratory Service (NHLS) database was searched to extract all PCR adenovirus-positive respiratory samples with the diagnosis of pneumonia, bronchiolitis or LRTI collected in children admitted to RCWMCH during the study period. Respiratory viruses were identified using SeeplexRV7 Detection assay (Seegene, Korea), which includes influenza A and B, human metapneumovirus, RSV, rhinovirus A, parainfluenza virus and adenovirus.
Clinical and laboratory data
The medical records of identified patients were retrieved and relevant data were recorded on a data capture form. Clinical and demographic information at the time of hospital admission for community-acquired AVP cases, or acquisition of adenovirus infection in hospital-acquired AVP cases, was collected. The presence of fever (temperature >38oC for >48 hours), diarrhoea, conjunctivitis and skin rash was recorded. AVP cases were classified into community acquired or hospital acquired. Community-acquired AVP was defined as AVP confirmed within 48 hours of hospital admission. Hospital-acquired AVP was defined as AVP acquired >48 hours after hospital admission when the presenting illness on admission was not pneumonia.
The HIV status of patients was classified as HIV-exposed but uninfected, HIV-infected or HIV-unexposed. In the HIV-infected group, CD4 count and percentage, HIV viral load and antiretroviral treatment details were recorded. The nutritional status of each child was determined by calculating the World Health Organization (WHO) weight-for-age z-score (WAZ score) using igrowup macro for Stata (WHO Anthro version 3.2.2; StataCorp, USA). Malnutrition was defined as a WAZ score of <-2. The presence of an underlying medical condition was recorded and categorised as cardiac, respiratory, renal, neurological, immune suppression, prematurity or other.
Routine laboratory investigations recorded were total white cell count (WCC), haemoglobin, C-reactive protein (CRP) and pro-calcitonin (PCT) at the time of hospital admission. The presence of significant laboratory-confirmed co-infections was documented and recorded as other respiratory viruses, positive blood culture, and tuberculosis (TB) (positive culture of Mycobacterium tuberculosis from any site).
Pneumonia severity was established according to the presence or absence of hypoxia at presentation (oxygen saturation <90% in room air), need for intensive care unit (ICU) admission and need for ventilator support. The ventilation modality and total duration of ventilation and ICU stay were recorded.
Outcome measures
Two outcome measures were examined: development of persistent lung disease (PLD) and in-hospital mortality. PLD was defined as the persistence of clinical signs of respiratory disease as documented in the medical records and/or need for supplemental oxygen beyond 30 days of hospital admission.
Data analysis
Data were collected and entered into EpiData Entry version 2.0 (Denmark). Thereafter statistical analysis was done using SPSS version 22.0 (IBM, USA). Data were tested for normality using the Shapiro-Wilks W-test. Continuous variables were expressed as means with standard deviations (SDs) for normally distributed variables or medians and interquartile ranges (IQRs) for non-normally distributed variables. The Mann-Whitney (7-test was used for comparing non-normally distributed measures and the t-test for normally distributed measures. Univariate analysis was conducted using Yates-corrected χ2 tests where cell values were <10. Multivariate logistic regression analysis was conducted for risk factors for death outcome using variables with a p-value of <0.05.
Approval to conduct the study was obtained from the Human Research Ethics Committee of the University of Cape Town (ref. no. 120/2013).
Results
Between 1 January and 31 December 2011, a total of 1 910 respiratory samples were submitted to the NHLS from RCWMCH for viral PCR testing. After excluding children without a diagnosis of pneumonia, 206 AVP cases were identified for analysis, of which 65 (31.6%) were co-infected with other respiratory viruses.
Demographic and clinical characteristics (Table 1)
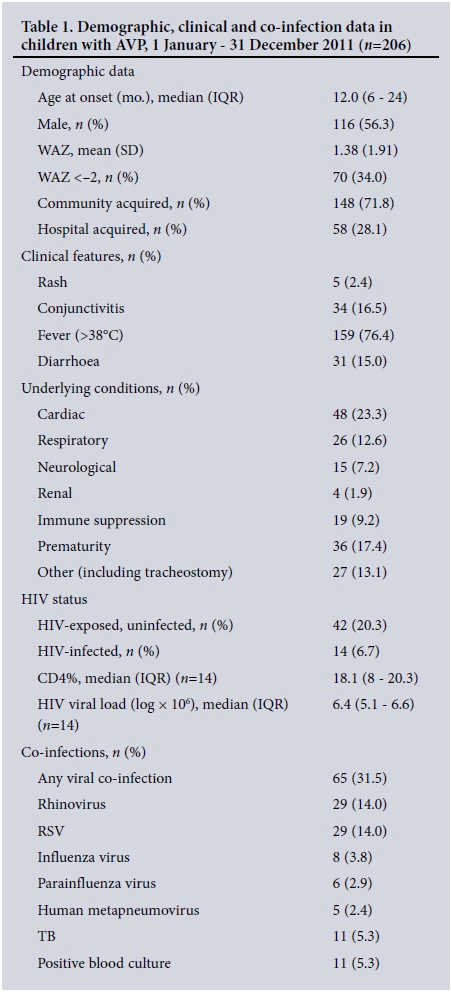
The median age was 12 months (IQR 6 - 24), with a slight male predominance. Of the study population, 34.0% (70/206) were malnourished and 71.8% (148/206) had community-acquired infections. Of the clinical features recorded, fever was the most common (76.4%). There was a high prevalence of underlying medical conditions, with 47.0% of participants having at least one underlying medical condition. All HIV-infected children (n=14) were previously undiagnosed; antiretroviral therapy was initiated in 12/14 (85.7%) during the same admission.
The mean WCC on admission was 15 χ 109/L (SD 8.0) and the mean haemoglobin concentration 11 g/dL (SD 2.0). The mean CRP and PCT were 20 mg/L (IQR 5 - 53, n=145) and 2.5 μg/L (IQR 1 - 12, n=10), respectively.
Pneumonia severity
Hypoxia was common, and present in 146/206 patients (70.9%); 76/ 206 (36.9%) needed ICU admission for respiratory support, of whom 54/76 (71.1%) were mechanically ventilated, 31/76 (40.8%) received continuous positive airway pressure ventilation, and 13/76 (17.1%) needed high-frequency oscillatory ventilation. The median stay in the ICU was 9 days (IQR 4 - 17). The median length of hospital stay was 10 days (IQR 3 - 21).
Outcomes Persistent lung disease
Most children recovered completely (168/ 206, 81.6%) and were discharged with no sequelae. Twenty-nine children (14.1%) developed PLD. Hypoxia (p=0.016) and ICU admission (p=0.002) were significantly associated with the development of PLD.
Viral co-infection (8/29 (27.6%) with PLD v. 57/177 (32.2%) without PLD; p=0.6) and TB (3/29 (10.3%) with PLD v. 8/177 (4.5%) without PLD; p=0.18) were not associated with PLD. Compared with children without PLD, children with PLD had a longer but non-significant median ICU stay (9 days v. 5 days; p=0.06) and a longer median hospital stay (41 days v. 9 days; p<0.001).
In-hospital mortality (Table 2)
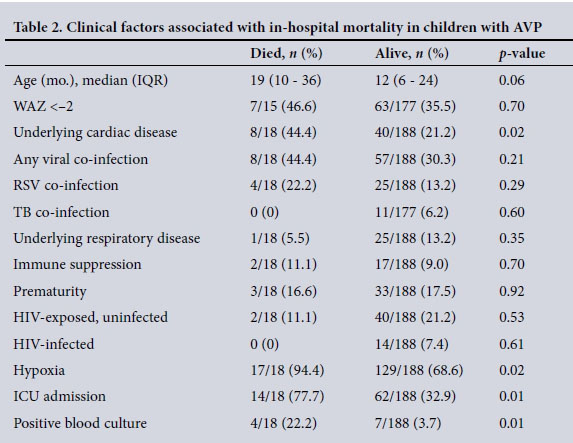
Eighteen of the 206 children (8.7%) died.
Hypoxia (p=0.02), ICU admission (p=0.01), underlying cardiac disease (p=0.02) and a positive blood culture (p=0.001) were associated with in-hospital mortality on univariate analysis (Table 2). Logistic regression analysis revealed that ICU admission (odds ratio (OR) 8.3, 95% confidence interval (CI) 2.3 - 29.0) and positive blood culture (OR 11.2, 95% CI 2.3 - 54.1) were independently associated with mortality. Underlying cardiac disease trended towards significant association with mortality (OR 2.88, 95% CI 0.98 - 8.5).
Discussion
This study investigated adenoviral respiratory infections in SA children, for which there were previously no data. In this study, adenovirus was isolated in 10.9% of all respiratory tract samples tested, highlighting the potential contribution of adenovirus to pneumonia aetiology in our setting. Furthermore, the study confirms previous reports of adenovirus causing severe pneumonia and significant morbidity in children, as supported by our data documenting 36.9% of patients needing ICU admission and 14.1% developing PLD.[14,15]
An underlying medical condition and malnutrition was found in almost half and one-third of patients, respectively, which is consistent with previous studies.[9] Prematurity and congenital cardiac diseases were the most common underlying conditions, highlighting the vulnerability of children with these conditions to AVP. Underlying cardiac disease was not an independent risk factor for death in our analysis. However, this finding must be interpreted with caution owing to small numbers. Adenovirus is directly cardiotoxic, and is a common cause of myocarditis and dilated cardiomyopathy in children.[16] Children with heart disease are therefore likely to be at increased risk of acquiring severe pneumonia with further myocardial impairment secondary to the direct effects of adenovirus infection on the myocardium.
In contrast to other studies, HIV exposure, HIV infection and malnutrition were not associated with mortality. The prevalence of HIV infection in this study was low (6.8%) and reflects effective strategies to prevent perinatally acquired HIV infection in SA.
A positive blood culture was also associated with increased mortality in this study. The synergistic effect produced by bacterial and viral co-infection resulting in more severe pneumonia has been described.[17]
The severity of AVP as measured by hypo-xia and need for ICU admission was associated with both development of PLD and death. This finding is consistent with previous studies looking at outcomes following adeno-viral infections. A meta-analysis of long-term sequelae of childhood pneumonia from mostly developed countries found that adenovirus was associated with the highest prevalence of sequelae; more than half of AVP cases resulted in long-term sequelae of chronic obstructive pulmonary disease.[13] Three studies from South America identified mechanical ventilation, prolonged oxygen requirement and prolonged hospitalisation as risk factors for the development of bronchiectasis obliterans.[10-12] In a study by Murtagh et al.,[10] 36% had chronic sequelae and 15% died. In our study, 14.1% developed PLD and 8.7% died. The lower incidence of PLD and death in our cohort compared with the Argentinian study could be explained by different study design, different definition of PLD or different epidemiology of adenovirus serotypes and disease spectrum in our setting.
Study limitations
This study has several limitations. Firstly, the retrospective search for eligible patients may not have represented all AVP cases, as testing for respiratory viruses was not routinely performed on all children admitted with pneumonia. There may have been a bias towards more severe pneumonia cases in this study, as clinicians were likely to request viral testing when pneumonia was more severe. Secondly, the attributable role of adenovirus infection in pneumonia was not established owing to the lack of controls. However, co-infection with other viruses or TB was not associated with a worse outcome. Furthermore, evidence of community-acquired pneumonia causality and increased adenovirus-associated morbidity in a paed-iatric intensive care setting in our population has been described.[6,15] Lastly, we were unable to identify adenovirus serotypes in this study, as testing for serotype is not routinely offered in our setting. Knowledge of serotype patterns may explain some observed differences in the spectrum of AVP disease severity, as described elsewhere.[9]
Conclusions
Adenovirus is potentially an important and under-recognised cause of severe pneumonia and PLD in young children in SA. Severe AVP necessitating ICU admission and a positive blood culture are associated with poor outcome. Early recognition of severe AVP is important to identify children at risk of developing chronic sequelae of AVP.
Acknowledgements. We thank Dr Marvin Hsiao of the NHLS, who helped with the database identification of eligible cases, and Ushma Galal for her invaluable assistance with the statistical analysis.
Funding. Non-funded research.
REFERENCES
1. Walker CL, Rudan I, Liu L, et al. Global burden of childhood pneumonia and diarrhoea. Lancet 2013;381(9875):1405-1416. http://dx.doi.org/10.1016/S0140-6736(13)60222-6 [ Links ]
2. Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med 2015;372(9):835-845. http://dx.doi.org/10.1056/NEJMoa1405870 [ Links ]
3. Berkley JA, Munywoki P, Ngama M, et al. Viral etiology of severe pneumonia among Kenyan infants and children. JAMA 2010;303(20):2051-2057. http://dx.doi.org/10.1001/jama.2010.675 [ Links ]
4. Rhedin S, Lindstrand A, Hjelmgren A, et al. Respiratory viruses associated with community-acquired pneumonia in children: Matched case-control study. Thorax 2015;70(9):847-853. http://dx.doi.org/10.1136/thoraxjnl-2015-206933 [ Links ]
5. Cohen C, Walaza S, Moyes J, et al. Epidemiology of viral-associated acute lower respiratory tract infection among children <5 years of age in a high HIV prevalence setting, South Africa, 2009 - 2012. Pediatr Infect Dis J 2015;34(1):66-72. https://dx.doi.org/10.1371/journal.pone.0117716 [ Links ]
6. Zar HJ, Barnett W, Stadler A, Gardner-Lubbe S, Myer L, Nicol MP. Aetiology of childhood pneumonia in a well vaccinated South African birth cohort: A nested case-control study of the Drakenstein Child Health Study. Lancet Respir Med 2016;4(6):463-472. http://dx.doi.org/10.1016/S2213-2600(16)00096-5 [ Links ]
7. Shi T, McLean K, Campbell H, Nair H. Aetiological role of common respiratory viruses in acute lower respiratory infections in children under five years: A systematic review and meta-analysis. J Glob Health 2015;5(1):010408. http://dx.doi.org/10.7189/jogh.05.010408 [ Links ]
8. Carballal G, Videla C, Misirlian A, Requeijo PV, Aguilar MdelC. Adenovirus type 7 associated with severe and fatal acute lower respiratory infections in Argentine children. BMC Pediatr 2002;2:6. https://dx.doi.org/10.1186%2F1471-2431-2-6 [ Links ]
9. Lee J, Choi EH, Lee HJ. Clinical severity of respiratory adenoviral infection by serotypes in Korean children over 17 consecutive years (1991-2007). J Clin Virol 2010;49(2):115-120. http://dx.doi.org/10.1016/j.jcv.2010.07.007 [ Links ]
10. Murtagh P, Giubergia V, Viale D, Bauer G, Pena HG. Lower respiratory infections by adenovirus in children: Clinical features and risk factors for bronchiolitis obliterans and mortality. Pediatr Pulmonol 2009;44(5):450-456. http://dx.doi.org/10.1002/ppul.20984 [ Links ]
11. Castro-Rodriguez JA, Daszenies C, Garcia M, Meyer R, Gonzales R. Adenovirus pneumonia in infants and factors for developing bronchiolitis obliterans: A 5-year follow-up. Pediatr Pulmonol 2006;41(10):947-953. https://dx.doi.org/10.1002/ppul.20472 [ Links ]
12. Colom AJ, Teper AM, Vollmer WM, Diette GB. Risk factors for the development of bronchiolitis obliterans in children with bronchiolitis. Thorax 2006;61(6):503-506. http://dx.doi.org/10.1136/thx.2005.044909 [ Links ]
13. Edmond K, Scott S, Korczak V, et al. Long term sequelae from childhood pneumonia: Systematic review and meta-analysis. PLoS One 2012;7(2):e31239. http://dx.doi.org/10.1371/journal.pone.0031239 [ Links ]
14. Castro-Rodriguez JA, Giubergia V, Fischer GB, et al. Postinfectious bronchiolitis obliterans in children: The South American contribution. Acta Paediatr 2014;103(9):913-921. http://dx.doi.org/10.1111/apa.12689 [ Links ]
15. Lonngren C, Morrow BM, Haynes S, Yusri T, Vyas H, Argent AC. North-South divide: Distribution and outcome of respiratory viral infections in paediatric intensive care units in Cape Town (South Africa) and Nottingham (United Kingdom). J Paediatr Child Health 2014;50(3):208-215. http://dx.doi.org/10.1111/jpc.12458 [ Links ]
16. Bowles NE, Ni J, Kearney DL, et al. Detection of viruses in myocardial tissues by polymerase chain reaction: Evidence of adenovirus as a common cause of myocarditis in children and adults. J Am Coll Cardiol 2003;42(3):466-472. http://dx.doi.org/10.1016/S0735-1097(03)00648-X [ Links ]
17. Wolter N, Tempia S, Cohen C, et al. High nasopharyngeal pneumococcal density, increased by viral coinfection, is associated with invasive pneumococcal pneumonia. J Infect Dis 2014;210(10):1649-1657. http://dx.doi.org/10.1093/infdis/jiu326 [ Links ]
 Correspondence:
Correspondence:
M Zampoli
m.zampoli@uct.ac.za
Accepted 24 October 2016.














