Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.107 n.2 Pretoria Feb. 2017
http://dx.doi.org/10.7196/samj.2017.v107i2.12012
IN PRACTICE
CLINICAL UPDATE
The prevalence of skin scars in patients previously given intramuscular diclofenac injections attending the Pain Clinic at Universitas Academic Hospital, Bloemfontein, South Africa
D TarloffI; G LamacraftII; G JoubertIII
IMB ChB, DA (SA), FCA (SA); Department of Anaesthesiology, Faculty of Health Sciences, University of the Free State, Bloemfontein, South Africa
IIMBBS, MRCP, FRCA, PhD; Department of Anaesthesiology, Faculty of Health Sciences, University of the Free State, Bloemfontein, South Africa
IIIBA, MSc; Department of Biostatistics, Faculty of Health Sciences, University of the Free State, Bloemfontein, South Africa
ABSTRACT
Intramuscular (IM) diclofenac rarely causes scarring (reported incidence <0.05%). Some patients attending the Pain Clinic at Universitas Academic Hospital, Bloemfontein, South Africa, presented with scars that had developed after IM diclofenac injections. We investigated the prevalence of scars in patients at the clinic and how the injections had been obtained. Patients attending the clinic over a period of 9 months who said they had received diclofenac (N=131) were included. Information was collected using a questionnaire and physical examination. Data obtained from 118 patients who were certain that they had received diclofenac were analysed. Ninety-three patients (78.8%) indicated they had not been warned about the possibility that a diclofenac injection could result in scarring. Scarring had occurred in 10 patients (8.5%). Two-thirds of the patients who had obtained diclofenac from a pharmacy had never had a prescription for it. Four patients had required medical treatment for an ulcer or abscess, of whom two had undergone surgery. The risk of skin lesions associated with IM diclofenac is higher than reported previously. Contrary to regulations, diclofenac injections were often dispensed to patients without a prescription.
The Pain Clinic at Universitas Academic Hospital in Bloemfontein, South Africa (SA), sees patients with various forms of chronic pain, most commonly back pain. Treating patients with low back pain often includes exposure of the patient's buttocks. Doctors in the clinic have occasionally noted disfiguring skin scars on the buttocks of patients that had developed after they had received intramuscular (IM) injections for their pain. These injections had been given elsewhere and were often reported by the patients to be IM diclofenac (Voltaren). It is not practice in this clinic to use or prescribe IM diclofenac. Some patients also reported that they had received IM diclofenac without a prescription, and that the injections had been administered by persons other than healthcare professionals.
IM diclofenac is on the South African Standard Treatment Guidelines and Essential Medicines List for treatment of adults with acute pain.[1] The current national contract for IM diclofenac in SA is for the period 1 June 2014 to 28 February 2017 and comprises ~6.77 million ampoules of IM diclofenac. The Free State Province had an annual usage rate of 115 700 ampoules for the period 1 December 2014 - 30 November 2015, while usage at Universitas Academic Hospital was 1 910 ampoules between 1 July 2014 and 30 June 2015.[2] This information was confirmed by personal communication with the assistant manager, Pharmaceutical Services, Medpharm, Free State Department of Health (9 November 2015) and a pharmacist at Universitas Academic Hospital (9 July 2015).
The extensive use of IM diclofenac in SA, where it is the only parenteral non-steroidal anti-inflammatory drug (NSAID) widely used in public hospitals, contrasts with the situation in other countries, where its use has been limited because of the ulceration, scarring and tissue loss that can result following its administration.[3,4]
Objective
The objective of this questionnaire-based study was to determine: (i) the prevalence of skin scars due to IM diclofenac in our patient community; (ii) how patients had obtained IM diclofenac; and (iii) who had administered it to them.
Methods
A prospective descriptive study was performed using a questionnaire and clinical examination. The study protocol was approved by the Ethics Committee of the Faculty of Health Sciences, University of the Free State (ref. no. 173/2013). All patients who attended the Pain Clinic at Universitas Academic Hospital over a 9-month period (1 December 2013 - 31 August 2014) were included in the study. Patients who attended the clinic more than once during this period were included for their first visit only.
Informed consent was obtained from each patient. Consent documents and information forms were available in the three main languages of the region, namely English, Afrikaans and Sesotho. Each patient who attended clinic during the study period was asked by the admitting nurse if they had ever received an IM diclofenac or Voltaren injection. If they answered 'yes', they were asked to participate in the study. The nurse kept a record of how many patients seen at the clinic answered 'yes' or 'no'.
Participants included in the study completed a questionnaire and underwent a non-invasive physical examination of the injection site by the doctor working in the clinic, who was either an anaesthetic consultant or a registrar. Information was collected confidentially. The folders were marked to ensure that each patient participated only once during the period of the study.
On physical examination of the participant, the following information was recorded: body mass index (BMI) of the patient; site of the intramuscular injection(s); and skin changes at the injection sites, such as scarring, ulcer formation or changes suggestive of a healed ulcer. When a scar was observed, its size was measured and any changes in colour and/or sensation (increased or decreased sensation) associated with the scar were noted. A photograph of the scar was taken as documentation, with the patient's consent.
A pilot study conducted on five patients during the month before the study period resulted in some items on the questionnaire being rephrased. The amendments to the questionnaire were approved by the Ethics Committee before commencement of the study. The results from the pilot study were not included in subsequent data analysis.
Statistical analysis
Data analysis was performed by the Department of Biostatistics, University of the Free State. Results were summarised by frequencies and percentages (categorical variables) and means and standard deviations (SDs) of percentiles (numerical variables).
Results
Two hundred and nineteen patients attended the Pain Clinic during the study period. When asked whether they had ever received a diclofenac or Voltaren injection, 131 patients (59.8%) answered 'yes' and were asked to participate in the study.
The mean age of the participants was 54.2 years (SD 12.1, range 23 - 80). The BMI was recorded in 127 participants, who had a median BMI of 29.2 kg/m2 (range 16.2 -56.6). Approximately two-thirds (66.4%) of the participants were female. Seventeen participants (13.0%) had diabetes mellitus. The median number of injections received by each participant was 8 (range 1 - 95).
One hundred and twenty-five participants (95.4%) stated in the questionnaire that the pain-relieving injection they had previously received had been diclofenac (Voltaren). The remaining 6 participants were unsure of the name of the drug injected (although they had initially said they thought they had received a diclofenac injection). Of the 125 participants who stated that the injection had been diclofenac, 94.4% (n=118) were 100% sure of the name of the injection, 4.0% (n=5) were 60 - 90% sure, and 1.6% (n=2) were less than 60% sure.
The data analysis was continued only for the 118 participants who indicated that they were 100% sure that the pain-relieving injection they had received had been diclofenac. Ten (8.5%) of these participants were identified as having a scar, of whom 5 had a BMI >30 kg/m2. Of the patients with diabetes, 18.8% had a scar, compared with 6.9% of patients without diabetes (p=0.14).
Of the 118 participants who were 100% sure that the pain-relieving injection had been diclofenac, 66 (55.9%) indicated that it had been administered by a general practitioner. Diclofenac injections had also been administered by a trained nurse (n=37, 31.4%) and a pharmacist (n=29, 24.6%), and in 6 cases (5.1%) by a family member with medical training (Fig. 1). In 3 cases (2.5%) the diclofenac injection had been administered by a family member without medical training, and in 3 cases (2.5%) it was self-administered.
When asked where the IM diclofenac had been obtained from (Fig. 2), 51.7% (n=61) of the participants indicated it had been obtained from a general practitioner and 37.3% (n=44) had obtained it from a private pharmacist. Other sources are also shown in Fig. 2.
In response to the question 'If you received the injection from a pharmacist, did you have a prescription?', 38 (66.7%) of the 57 participants who had obtained the diclofenac from either a private or a hospital pharmacy said that they had never had a prescription for it (Table 1); these 38 participants lived a median of 3 km from a medical doctor (range 1 - 32 km). In response to the question 'Have you ever been warned that you may get a skin scar from IM diclofenac injection?', 93 participants (78.8%) indicated that they had not been warned.

Most injections had been given recently, with 80 participants (67.8%) indicating that they had received their last IM diclofenac injection during the preceding year, 22 (18.6%) in the past 5 years and 16 (13.6%) >5 years previously. Of the 10 participants who had developed a scar from the diclofenac injection, 7 had received the injection within the preceding year, 1 within the preceding 5 years, and 2 >5 years previously.
As shown in Table 2, the most common complication noted after the diclofenac injection reported by participants who were 100% sure that they had received IM diclofenac was pain (n=27, 22.9%), followed by pruritus (n=23, 19.5%) and erythema (n=19, 16.1%) at the site of injection. Scarring at the injection site was initially reported by only 2 patients, although 10 patients were subsequently identified on the medical examination as having a scar.
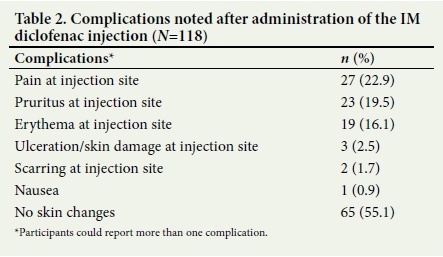
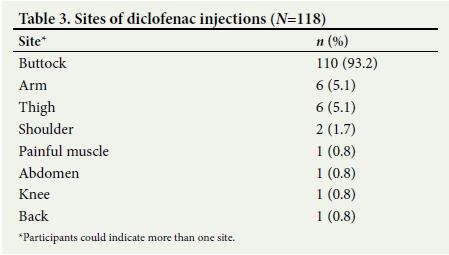
Of the participants who could provide information regarding the site of injection, the majority indicated that the IM diclofenac had been administered into the buttocks (n=110, 93.2%). Other sites included the arm, thigh and shoulder, directly into the painful muscle, and the abdomen, knee and back, as shown in Table 3.
The patients with scars had received a median of 15 injections (range 2 - 95), compared with a median of 6 injections (range 1 - 50) for the patients without scars (p=0.08). Of the 10 participants who had scars associated with IM diclofenac injections (Table 4), all had scars on the buttocks and 2 also had scars on the upper arm (deltoid region). No scars were observed elsewhere. Three participants experienced ongoing hyperalgesia or allodynia over the site of the scars, and 2 had decreased sensation at the site of the scar. The size of the scars ranged from 5 χ 5 mm2 to 30 χ 40 mm2. Skin colour changes, observed in 4 participants, were described as either a blue discolouration or hyperpigmentation. Panels A, B and C in Fig. 3 show examples of the skin lesions associated with IM diclofenac injections observed in the participants in the study. Two of the 10 participants with confirmed scarring (Table 4) and needing medical attention had also required surgical treatment in the form of incision and drainage of an abscess.

Discussion
Diclofenac is an NSAID with analgesic, anti-inflammatory and antipyretic properties. NSAIDs are used to treat a wide spectrum of ailments including musculoskeletal or joint pain, soft-tissue injury, acute gout, renal colic and postoperative pain. Parenteral administration is favoured for acutely painful conditions, with the IM route often used as a stat injection. When given intravenously, diclofenac has to be given as a slow infusion, and thrombophlebitis may result.'51
Skin complications from IM diclofenac have been reported previously. The findings of a 25-year analysis of complications due to IM diclofenac injections published in the BMJ in 2003[6] (response to Wright et al.[4]), reviewing the Novartis Global Safety Database of spontaneous and clinical trial reports for diclofenac ampoules from 1978 to 2003, revealed 115 reports of injection site necrosis, 37 reports of site abscess, 11 reports of injection site reaction, 6 reports of necrotising fasciitis and 2 cases of necrotising myositis. Pain on injection was only reported in 9 of these recorded cases.[6]
The review[6] compared these complications with the frequency that IM diclofenac was used. Over the 25-year period it was estimated that >100 million units of IM diclofenac were used (4 million per annum) in the UK. The incidence of lesions associated with IM diclofenac could therefore be regarded as diminutive. The review[6] also reported that for 10 167 IM injections of diclofenac the incidence of abscess was 0.05%, necrosis at the injection site 0.02% and pain at the injection site 5.6%.
The results of the present study show a much higher risk of scars from IM diclofenac injection, with an 8.5% prevalence of scarring. The markedly different results from the Novartis Database could indicate a considerable under-reporting of skin lesions resulting from diclofenac injections.
While skin scars can be unsightly or painful (30% of the scars in this study were associated with hyperalgesia or allodynia), the skin complications of IM diclofenac can have more serious results. A particularly severe skin complication of IM diclofenac is Nicolau syndrome (embolia cutis medicamentosa). This is a rare occurrence that has followed IM injections of diclofenac and other common drugs, including other NSAIDs, corticosteroids and penicillin. The clinical presentation is typically pain around the injection site, followed by erythema, liveoid patch, haemorrhagic patch and finally necrosis of the skin, subcutaneous fat and muscle tissue. Several case reports of Nicolau syndrome following intramuscular diclofenac have been published, with severe scarring and even death as an outcome.[6-8]
Nicolau syndrome was first described in 1925 following the use of bismuth salts for the treatment of syphilis. This phenomenon has now been related to the administration of a variety of drugs such as corticosteroids, local anaesthetics and antihistamines.[7] The pathogenesis of Nicolau syndrome is not well understood, but probably involves acute vasospasm, arteritis and thromboembolic occlusion of small arteries resulting in end-organ damage. Diclofenac is associated with the vasoconstrictive phenomenon as it inhibits prostaglandin synthesis due to cyclooxygenase inhibition, and can therefore cause Nicolau syndrome or milder forms of skin necrosis.[9]
The manufacturer's instructions for IM diclofenac are very detailed and explicit, clearly stating that the injection should be administered by deep intragluteal injection (not in other sites such as the leg or arm), using strict aseptic techniques and not exceeding the recommended dose. It also states that subcutaneous and intravascular injection must be avoided to prevent skin scarring.[6,8] Furthermore, the Z-track method of IM injection, as described and illustrated by Lie et al.,[8] can minimise subcutaneous irritation by blocking the needle track after injection and should be adopted as a standard procedure. According to this method, with the skin, subcutaneous fat and muscle in a normal position, the skin and subcutaneous layer should be pulled down 1 cm to de-align with the underlying muscle. The needle should be inserted at 90o to administer the injection into the muscle and then withdrawn. When the finger is released to break the needle track, the drug is trapped inside the muscle.[8]
In SA, IM diclofenac is a schedule 3 drug and should only be dispensed to patients with chronic pain with a prescription by a medical doctor. However, diclofenac may be dispensed by a pharmacist without a prescription as a schedule 2 drug (Medicines and Related Substances Act 101 of 1965, Section 22A(5)(a)) for the emergency treatment of acute gout and post-traumatic injuries for 5 days only.'10,111 IM diclofenac should only be administered by a trained healthcare professional. A pharmacist may administer IM diclofenac if he or she has received the necessary training and is competent in the technique. Our findings show that IM diclofenac was inappropriately dispensed by some pharmacists, as two-thirds of the patients who were dispensed IM diclofenac from a pharmacy received it without a prescription - a matter of concern, because it implies that some pharmacists dispense this drug illegally. This cannot be excused by claiming that these individuals were not able to visit a doctor to obtain a prescription for the injection because they lived in remote, rural areas, as they lived on average only a few kilometres away from a medical doctor.
This study did not investigate whether the participants who received the injection from a pharmacist received it from one trained in the correct technique. This may be investigated in a further study. A concerning finding was that several participants received IM diclofenac injections from an unauthorised person or even administered the injection themselves. This suggests that when IM diclofenac is dispensed, there should be tighter regulations to ensure that the patient only receives the injection from an appropriately trained healthcare professional. Incorrect administration was confirmed by the observation that two of the scars from IM diclofenac were on the upper arms of the participants.
Before receiving IM diclofenac, patients should be warned of the potential for skin scarring, which is clearly stated in the package insert. The scars are probably more common than previously thought, as 8.5% of the participants in this study who had received IM diclofenac had scars from this injection. Despite this cautionary note, 78.8% of the study population indicated that they had never been warned about skin scarring as a potential side-effect. Medical professionals should warn patients against this side-effect when prescribing IM diclofenac, as there may be legal implications if scars develop.
The skin scars from IM diclofenac given at the correct intragluteal site may not be visible to the affected individual and therefore not necessarily of concern to them. Some people, however, do not wish to have scars on their buttocks for their own personal reasons, and patients should therefore be informed of the possible risk before IM diclofenac is prescribed. In this study, some scars were large and some were chronically painful, demonstrating that there is notable morbidity associated with these scars.
Patients with diabetes are at risk of developing infections after any invasive procedure, and an infected IM diclofenac injection site increases the risk of scar formation. We suggest that diabetes mellitus should be considered a risk factor for developing scars from IM diclofenac. The prevalence of skin scars in diabetic participants was more than double that in non-diabetics, although the difference was not statistically significant. A larger study is necessary to determine the role of diabetes as a risk factor.
Study limitations
A major limitation of this study is that it relied on the memory of the participants completing the questionnaire. Recall bias may have exaggerated symptoms. They may not accurately have recalled all the IM diclofenac injections they had received in the past, or other related facts, or may have had an injection with another medication and erroneously said that it was diclofenac. Some participants did not answer all the questions, and data were lost in this small number of cases.
Conclusions
A larger prospective study to investigate the risk of skin scars from IM diclofenac injections is required. Until this has been done, patients should be warned that they may develop a skin scar after IM diclofenac, as stated in the package insert. This warning should be documented as having been given, and oral diclofenac should be prescribed where possible.
Acknowledgements. We thank Dr Daleen Struwig, medical writer/editor, Faculty of Health Sciences, University of the Free State, for technical and editorial preparation of the manuscript.
REFERENCES
1. National Department of Health, South Africa. Standard Treatment Guidelines and Essential Medicines List for South Africa. Hospital level adults. 2012 edition. http://www.kznhealth.gov.za/pharmacy/edladult_2012.pdf (accessed 30 June 2016). [ Links ]
2. South African Government. Supply and delivery of small volume parenteral and insulin devices to the Department of Health for the period 1 June 2014 to 28 February 2017. HP06-2014 SVP. Government Tender Bulletin 2012;564(2732). http://www.gov.za/sites/www.gov.za/files/ten_2732.pdf (accessed 30 June 2016). [ Links ]
3. World Health Organization. 19th WHO Model List of Essential Medicines (April 2015). www.who.int/medicines/publications/essentialmedicines/EML2015_8-May-15.pdf (accessed 30 June 2016). [ Links ]
4. Wright PJ, English PJ, Hungin APS, Marsden SNE. Managing acute renal colic across the primary-secondary care interface: A pathway of care based on evidence and consensus. BMJ 2002;325(7377):1408-1412. http://dx.doi.org/10.1136/bmj.325.7377.1408 [ Links ]
5. Chung CH. The use of injectable non-steroidal anti-inflammatory drugs in local accident and emergency practice. Hong Kong J Emerg Med 2002;9(2):65-71. http://hkcem.com/html/publications/Journal/2002-2/065-071.pdf (accessed 30 June 2016). [ Links ]
6. O'Sullivan DP, Collins J. Intramuscular diclofenac: 25 year worldwide safety perspective is vital to consider. BMJ 2002;325:1408. http://dx.doi.org/10.1136/bmj.325.7377.1408 [ Links ]
7. Panariello L, Ayala F. Nicolau syndrome following intramuscular diclofenac injection: A cas e report. Dermatol Ther 2008;21(Suppl 1):S10-S12. http://dx.doi.org/10.1111/j.1529-8019.2008.00195.x [ Links ]
8. Lie C, Leung F, Chow SP. Nicolau syndrome following intramuscular diclofenac administration: A case report. J Orthop Surg (Hong Kong) 2006;4(1):104-107. http://dx.doi.org/10.1177/230949900601400123 [ Links ]
9. Park HJ, Kim MS, Park NH, Jung SW, Park SI, Park CS. Sonographic findings in Nicolau syndrome following intramuscular diclofenac injection: A case report. J Clin Ultrasound 2011;39(2):111-113. http://dx.doi.org/10.1002/jcu.20743 [ Links ]
10. South African Legal Information Institute (SAFLII). Medicines and Related Substances Act 101 of 1965 - Regulations and Notices - Government Notice R510. http://www.saflii.org/za/legis/consol_reg/marsa101o1965rangnr510723/ (accessed 10 January 2017). [ Links ]
11. Medicines Control Council of South Africa. Scheduling of substances for prescribing by authorised prescribers. http://www.mccza.com/Publications/DownloadDoc/83 (accessed 28 August 2016). [ Links ]
 Correspondence:
Correspondence:
D Tarloff
debstarloff@yahoo.com
Accepted 24 October 2016.














