Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SAMJ: South African Medical Journal
versión On-line ISSN 2078-5135
versión impresa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.106 no.8 Pretoria ago. 2016
http://dx.doi.org/10.7196/samj.2016.v106i8.10136
IN PRACTICE
HEALTHCARE DELIVERY
A successful lifestyle intervention model replicated in diverse clinical settings
S MarkI; S du ToitII; T D NoakesIII; K NordliIV; D CoetzeeV; M MakinV; S van der SpuyV; J FreyV; J WortmanV
IMSc, PhD; Approach Analytics, Nanaimo, British Columbia, Canada
IIMD; Valemount Health Center, British Columbia, Canada
IIIMD, DSc; Department of Human Biology, Faculty of Health Sciences, University of Cape Town; Sports Science Institute of South Africa, Cape Town, South Africa
IVMD; Valemount Health Center, British Columbia, Canada
VMD; Omineca Medical Clinic, Vanderhoof, British Columbia, Canada
ABSTRACT
Lifestyle interventions (Lis) can treat metabolic syndrome and prevent type 2 diabetes mellitus, but they remain underutilised in routine practice. In 2010, an LI model was created in a rural primary care practice and spread with few resources to four other rural practices. A retrospective chart review evaluated changes in health indicators in two practice environments by following 372 participants, mainly women (mean age 52 years). Participants had a mean body mass index of 37 kg/m2 at baseline and lost an average of 12% of their initial body weight as a result of the intervention. Among participants at the first intervention site for whom cardiometabolic data were available, the prevalence of metabolic syndrome decreased from 58% at baseline to 19% at follow-up. Taken as a whole, our experience suggests that Lis are feasible and deliver meaningful results in routine primary care practice.
Lifestyle interventions (Lis) can treat metabolic syndrome and reduce the incidence of type 2 diabetes mellitus (T2DM) in high-risk individuals.1 However, realising the health benefits of Lis in routine clinical practice remains elusive.2 In January 2010, an LI model was created in a rural primary care practice and spread to four other rural communities. We present changes in health indicators among participants in two physician-led interventions.
Methods
Ethics approval was obtained from the Northern Health Authority and the University of British Columbia (Ref. no. H10-02573), Canada. The intervention was open to both individuals wanting to lose weight and those interested in a non-pharmacological approach to managing insulin resistance. The foundation of the intervention model was group medical visits, with 15 - 25 participants overseen by a clinical facilitator.
The clinically facilitated meetings featured a presentation germane to living a healthier lifestyle, such as sugar addiction, medication management, maintaining adherence while on vacation, etc. The remainder of the meeting time was used to answer participants' questions and address experiences relating to living a healthier lifestyle. At the first site (S1), programme length was determined by participants' progress towards their health goals. At the second site (S2), the intervention was 3 months in length. Individuals requiring additional support were encouraged to form peer-led support groups on their own initiative.
A quality improvement process whereby various lifestyle prescriptions were tested with different groups was used to refine the intervention at S1. Results from these non-randomised 'trials' were tracked using local electronic medical record data. The outcome of this process was an intervention featuring a two-stage diet programme. The weight-loss diet restricted calories to approximately 1 100 and 1 500 kcal/day for women and men, respectively; participants were instructed to avoid foods containing sugar and other refined carbohydrates, in addition to restricting the consumption of dietary fat. To assist in appetite control, participants were instructed not to undertake moderate or vigorous physical activity until they had reached their weight-loss goal. After reaching their target weight, a high-fat diet was used for weight maintenance. The use of a high-fat diet was predicated on the high prevalence of insulin resistance in the patient population and favourable changes in multiple health indicators in randomised trials of up to 2 years' duration in such populations.3 Foods consumed on the maintenance diet included beef, poultry, fish, eggs, oils, moderate amounts of hard cheeses, and small amounts of nuts, nut butters, seeds and berries.
Measurements
Height was measured using a stadiometer, with participants wearing no shoes. Waist circumference was measured with a flexible tape measure and with the help of another participant. Weight was measured at every group visit with participants wearing light indoor clothing. Participants completed the PHQ-9 questionnaires to assess their mood. Scores on the PHQ-9 range from 0 (absence of depressive symptoms) to 27 (severe depressive symptoms). A score of >10 on the PHQ-9 was used to indicate depression.
Evaluation rationale, data extraction and statistical analyses
Following the success of the intervention at S1, the intervention model spread to four other rural communities. The intervention was created in 2010 by a physician (SDT) working in a service contract environment and was adopted into the practices of four fee-for-service physicians, a nurse practitioner and two registered nurses. The initial evaluation was planned to document health changes at S1, but owing to the unanticipated spread of the intervention, weight-loss results were included in the evaluation from four fee-for-service physicians.
The clinical and community observations from the other sites, which have not been formally evaluated, were consistent with the health improvements reported here.
Data were extracted from an electronic medical system during the most active periods of the interventions; this was early 2010 to 2011 for S1, while for S2 anthropometric data were retrieved beginning in early 2012. For S1, where cardiometabolic data were available, baseline data were sought no earlier than January 2008. The time between the first and last weighing was used to define the time spent in the LI by participants at S1, as participants were weighed at each meeting. Intensity of participation in the LI was calculated by dividing the months in the programme by the number of visits. We used International Diabetes Federation crite-ria4 to classify participants as having metabolic syndrome (MetS). A haemoglobin A1c (HbA1c) measurement was used to indicate the presence of diabetes, in accordance with local clinical protocols.
Differences between anthropometric and biochemical variables were calculated, and paired f-tests were used to assess whether baseline values were statistically different to those at follow-up. Owing to the small sample size of individuals with HbA1c measurements (n=18), the Wilcoxon signed-rank test was used to test whether follow-up values were significantly different from baseline. We used multivariable analysis to examine the predictors of changes in body mass index (BMI) between baseline and follow-up in participants at S1. All statistical analyses were conducted on SAS version 9.2(SAS Institute, USA).
Results
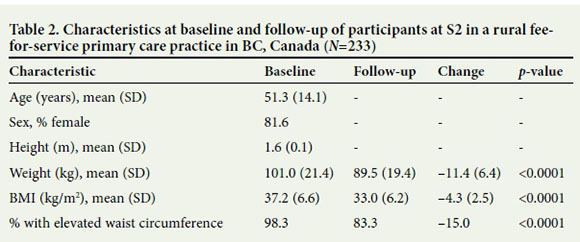
This study documented the creation and replication of a successful LI in rural British Columbia (BC). We evaluated health improvements among 372 participants at two physician-led interventions in a service contract (S1) and a fee-for-service practice context (S2); 139 participants were evaluated at S1 and 233 at S2, which began 2 years after the creation of the LI model at S1 (Tables 1 and 2). Participants at both sites were mainly women (~80%), with a mean age of 52 (standard deviation (SD) 13) years and 51 (SD 14) years for S1 and S2, respectively. Participants ranged in age from 16 to 85 years. Additional measures were available from participants at S1, including cardiometabolic indicators (n=119) and mood scores (n=111). More than 90% of participants had a high waist circumference, while the average baseline BMI was 37 kg/ m2. Consistent with the high prevalence of obesity, 57.6% (80/139) of participants began the intervention at S1 with MetS while 12.9% (18/139) had T2DM.

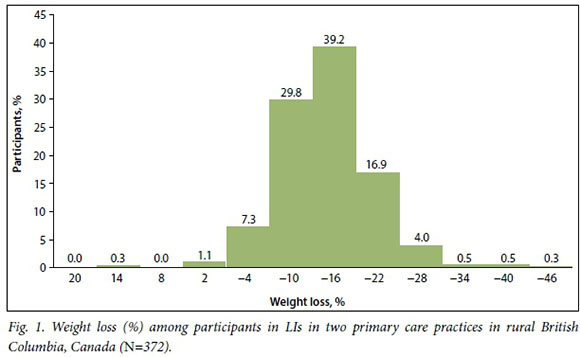
Participants in these LIs had unusually large improvements in health, particularly given the real-world contexts of the interventions. For example, 372 participants had weight loss of >12% (Fig. 1), while in the Diabetes Prevention Program, a well-resourced trial, the weight-loss goal was 7% of initial body weight. Among other studies examining LIs in routine practice, average weight loss was 3 - 5% at year 1.2
Consistent with the considerable weight loss, participants at S1 showed marked improvements in their cardiometabolic profile. For example, blood triglyceride concentrations, measured among 119 participants at S1, decreased by 34%, probably a reflection of the reduced intake of starches and sugars.3 Among the 18 individuals with T2DM in the LI at S1, there was a mean decrease in HbA1c of 0.5%, a figure that fails to account for any reductions in pharmaco-therapy, which were not documented in this report. The extent of the changes in cardio-metabolic indicators that were measured in this study are therefore a conservative estimate of the health improvements, as participants experienced reductions in the use of insulin and oral hypoglycaemic, antihypertensive and cholesterol-lowering agents. To the intervention participants, the reductions in pharmacotherapy were an empowering 'side-effect' of the intervention, and for the clinicians administering the intervention, use of this therapeutic approach improved control of hyperglycaemia, hypertension and dyslipidaemias.
Similar to the findings of others,5,6 we documented improvements in mood among participants in the intervention at S1 (n=111). Among the 32 participants with mood scores indicative of depression (PHQ-9 score >10), the mean decrease in score was 7.0 (SD 5.2). It was not possible to separate the effect of participating in group sessions from the physiological effects of the LI, as both these exposures are probably associated with improvements in mood. The mood improvements associated with weight loss may be attributable to reductions in patho-physiological processes such as inflammation and hypothalamic-pituitary-adrenal axis activation that are common to both insulin resistance and mood disorders.7
We examined the predictors of weight change among participants at S1 using multivariable analysis (Table 3). We found that each visit per month increase in the LI was associated with a 0.7 kg/m2 greater loss in BMI after controlling for sex, age, baseline BMI and time spent in the programme. The importance of intensity of participation was reaffirmed in regression analysis by using change in weight per month as the dependent variable. These findings highlight the importance of group support in achieving therapeutic goals. Moreover, the clinical observation prior to the creation of the LI model was that one-on-one lifestyle counselling was less effective in producing lifestyle changes than participation in support groups. The effectiveness of support groups in the context of LIs has been documented previously8 and may indicate the contribution of food addictions to these conditions.9

Conclusions
We documented the creation of an LI model and the replication of this intervention in different rural practices. This intervention was a powerful wellness tool, empowering not only patients101 and physicians but the rural communities, which can be burdened with a high prevalence of chronic disease.
The intervention model documented in this study differed from the consensus prescription for LIs. For example, participants in this intervention were counselled to restrict moderate to vigorous physical activity while on the weight-loss diet; in contrast, in two highly cited randomised trials, participants were encouraged to undertake 1501 and 17511 minutes per week of moderate physical activity, respectively. More controversially, our intervention used a high-fat diet for weight maintenance, while in the Diabetes Prevention Program and the Look AHEAD trials, participants were counselled to avoid consuming foods rich in
dietary fat. These conflicting prescriptions allude to a state of uncertainty that exists with regard to the optimal prescription for LIs for individuals with insulin resistance. This situation persists despite decades-old findings by Reaven (Garg et al.12) showing that insulin resistance is fundamentally a disorder of carbohydrate metabolism.
Given the magnitude of the obesity and diabetes pandemics, there is a public health imperative to provide practitioners with evidence that supports effective interventions. While well-resourced randomised trials are powerful analytical tools, rigorous trials take decades to yield results and are arguably prohibitively expensive.13 Moreover, study results often lack generalisability to routine practice.14 In contrast, the quality improvement process used in this study was not only powerful, as evidenced by the replication of the intervention model at four different practice sites, but offers a more expeditious way to spread effective interventions for obesity and insulin resistance.
Despite the rigour of our quality improvement process, our efforts to communicate the merits of this intervention to health system administrators met with a frustrating lack of uptake. This is not surprising, given that the research literature has many competing 'solutions' for the epidemics of obesity and diabetes,15 many of which are difficult to falsify.16 To support health administrators in making evidence-based decisions, a broader set of data sources could be used to evaluate health system interventions such as that documented here. For example, the Institute of Health Improvement recommends using indicators that measure patient satisfaction, health system cost and population health status,17 complementing data from physician records. A broader set of health system indicators combined with longer-term follow-up of intervention participants would enable evidence-based health system decision-making in a climate of fiscal restraint.
Taken as a whole, our evidence suggests that a timely response to the obesity and diabetes pandemics requires a critical rethink not only of the current evidence base underpinning LIs, but also of the systems with which evidence is generated and integrated into health system practice.
Conflicts of interest. SDT, KN, DC, MM, SVDS and JF have no conflicts of interest to declare. SM is the founder of a sole proprietorship, Approach Analytics, providing analytical support to clinical and public health initiatives. JW is on the Scientific Advisory Board for Atkins Nutritionals Inc. and has accepted honoraria and travel expenses to attend meetings. TN is the author of the books Lore of Running and Waterlogged and co-author of The Real Meal Revolution, Raising Superheroes and Challenging Beliefs. All royalties from the sales of The Real Meal Revolution and Raising Superheroes and related activities are donated to the Noakes Foundation, of which he is the chairman and which funds research on insulin resistance, diabetes and nutrition as directed by its Board of Directors. Money from the sale of other books is donated to the Tim and Marilyn Noakes Sports Science Research Trust, which funds the salary of a senior researcher at the University of Cape Town, South Africa. The research focuses on the study of skeletal muscle in African mammals with some overlap to the study of type 2 diabetes in carnivorous mammals and of the effects of (scavenged) sugar consumption on free-living (wild) baboons.
References
1. Orchard TJ, Temprosa M, Goldberg, R, et al. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: The Diabetes Prevention Program Randomized Trial. Ann Intern Med 2005;142(8):611-619. DOI:10.7326/0003-4819-142-8-200504190-00009 [ Links ]
2. Kahn R, Davidson MB. The reality of type 2 diabetes prevention. Diabetes Care 2014;37(4):943-949. DOI:10.2337/dc13-1954 [ Links ]
3. Shai I, Schwarzfuchs D, Henkin Y, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med 2008;359(3):229-241. DOI:10.1056/NEJMoa0708681 [ Links ]
4. Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120(16):1640-1645. DOI:10.1161/circulationAHA.109.192644 [ Links ]
5. McClernon FJ, Yancy WS, Eberstein JA, Atkins RC, Westman EC. The effects of a low-carbohydrate ketogenic diet and a low-fat diet on mood, hunger, and other self-reported symptoms. Obesity (Silver Spring) 2007;15(1):182-187. DOI:10.1038/oby.2007.516 [ Links ]
6. Brinkworth GD, Noakes M, Buckley JD, Keogh JB, Clifton PM. Long-term effects of a very-low-carbohydrate weight loss diet compared with an isocaloric low-fat diet after 12 mo. Am J Clin Nutr 2009;90(1):23-32. DOI:10.3945/ajcn.2008.27326 [ Links ]
7. McIntyre RS, Soczynska JK, Konarski JZ, et al. Should depressive syndromes be reclassified as 'metabolic syndrome type II'? Ann Clin Psychiatry 2007;19(4):257- 264. DOI:10.1080/10401230701653377 [ Links ]
8. Jaber R, Braksmajer A, Trilling JS. Group visits: A qualitative review of current research. J Am Board Fam Med 2006;19(3):276-290. DOI:10.3122/jabfm.19.3.276 [ Links ]
9. Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: Behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev 2008;32(1):20-39. DOI:10.1016/j. [ Links ]
10. Lavoie C. Gestational diabetes: Poke, pee, and eat your carbs. Can Fam Physician 2011;57(7):756-757. [ Links ]
11. Look AHEAD Research Group, Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369(2):145-154. DOI:10.1056/NEJMoa1212914 [ Links ]
12. Garg A, Bantle JP, Henry RR, et al. Effects of varying carbohydrate content of diet in patients with non-insulin-dependent diabetes mellitus. JAMA 1994;271(18):1421-1428. DOI:10.1001/jama.1994.03510420053034 [ Links ]
13. Howard BV, van Horn L, Manson JE, et al. Low-fat dietary pattern and risk of cardiovascular disease: The Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006;295(6):655-666. DOI:10.1001/jama.295.6.655 [ Links ]
14. Rothwell PM. External validity of randomised controlled trials: 'To whom do the results of this trial apply?' Lancet 2005;365(9453):82-93. DOI:10.1016/S0140-6736(04)17670-8 [ Links ]
15. Taubes G. Prosperity's plague. Science 2009;325(5938):256-260. DOI:10.1126/science.325_256 [ Links ]
16. Ioannidis JP. Why most published research findings are false. PLoS Med 2005;2(8):e124. DOI:10.1371/journal.pmed.0020124 [ Links ]
17. Berwick DM, Nolan TW, Whittington J. The triple aim: Care, health and cost. Health Aff 2008;27(3):759-769. DOI:10.1377/hlthaff.27.3.759 [ Links ]
 Correspondence:
Correspondence:
S Mark
sean@approachanalytics.com
Accepted 6 June 2016.














