Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.106 n.8 Pretoria Aug. 2016
http://dx.doi.org/10.7196/SAMJ.2016.V106I8.11225
CME
Surgical management of spasticity
J M N EnslinI; A G FieggenII
IBPhysT, MB ChB, FCNeurosurgery (SA), MMed (Neurosurg); Division of Neurosurgery, Red Cross War Memorial Children's Hospital, and Constantiaberg Mediclinic, Cape Town, South Africa
IIMB ChB, MSc, MD, FCNeurosurgery (SA); Department of Neurosurgery, Faculty of Health Sciences, University of Cape Town, and Division of Neurosurgery, Red Cross War Memorial Children's Hospital, Cape Town, South Africa
ABSTRACT
The management of patients with cerebral palsy and other causes of spasticity is a challenge to an entire rehabilitation team and to caregivers. In South Africa, neurosurgeons have had limited involvement in this field owing to a perceived lack of options, leaving the care of these patients largely in the hands of paediatric neurologists and orthopaedic surgeons. A committed team-based approach, where a neurosurgeon is part of the decision-making process, can however significantly improve functional outcomes in patients with spasticity. Key to the evaluation and therapeutic decision-making is the focus on function - not only the range of movement or the presence of spasticity. Some techniques can completely remove spasticity and contractures, but these mostly leave a patient with more functional impairment than they had before the surgery. With the careful combination of botulinum toxin injections and oral baclofen, these patients, who may benefit from further orthopaedic and neurosurgical procedures, can be identified and helped in reducing the function-limiting spasticity. With the emphasis on function as an individualising factor, significant improvements may follow minor interventions, e.g. performing a surgical procedure to allow reduced hip adductor spasticity, thereby allowing improved hygiene and less pain in a child in whom it was previously not possible to abduct the hips enough to change a nappy. Functional improvement does not necessarily equate to walking. We describe the process of evaluating patients with spasticity and outline the surgical decision-making process that helps towards an individualised therapeutic strategy in managing this challenging group of patients.
Spasticity is defined as an increase in muscle tone that is elicited by a velocity-dependent stretching of the muscles. It is part of the upper motor neuron syndrome of muscle weakness and is worst in antigravity muscles.[1,2] The most common causes of spasticity are:
• cerebral palsy (spasticity is a dominant feature in 80% of all patients with cerebral palsy)[3]
• stroke
• traumatic brain injury
• spinal cord injury
• multiple sclerosis
• hereditary spastic paraparesis
• degenerative neuromuscular diseases.
Management of spasticity should follow a planned, stepwise process of gradual escalation of therapy; only when the preceding step is shown to be ineffective, should more invasive options be considered. It is especially important to remember that the presence of spasticity in itself is not an indication for surgical management - only when spasticity becomes troublesome does surgery become an option. Criteria used to consider surgical management options are:
• spasticity that impairs function in a patient, e.g. difficulty with walking owing to spastic hamstring muscle group and triceps surae muscles
• spasticity that causes significant pain to the patient
• spasticity that makes caring for the patient troublesome, e.g. adductor spasticity, which makes it difficult to change a child's nappy; or a debilitated stroke patient
• spasticity may lead to irreversible deformities of the skeletal system, such as hip dislocations or abnormal degeneration of hip and knee joints owing to asymmetrical weight-bearing on the joints.
Pathophysiology of spasticity
Over the past century there has been little progress in the understanding of the pathogenesis of spasticity. Concepts developed by
Sherrington[4] in his Nobel prize-winning work in 1932 still hold true today. Sherrington described the concept of reciprocal innervation and noted that the muscle is under inhibitory and excitatory control of the central nervous system. Key to his understanding of spasticity was loss of this control owing to injury to the spinal cord or brain. Loss of inhibitory control from the brain via the spinal cord leads to uninhibited excitation of the monosynaptic spinal reflex arc.[5] The muscles, therefore, fire on their own, which causes spasticity. A basic representation of this reflex arc is shown in Fig. 1.
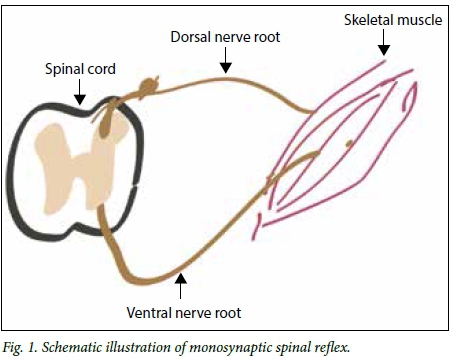
Inhibition of the corticospinal tract is generally thought to cause spasticity, but over the years it has been noted that there are many examples of corticospinal tract injuries where patients do not present with spasticity, but reduced tone. It is thought that the reticulospinal tract, which has a modulation effect via interneurons on the afferent signal to muscles, may play a more significant role in the pathogenesis of spasticity, and loss of reciprocal inhibition after spinal cord injuries or cerebral injuries may lead to spasticity.[5] From the many theories of the causes of spasticity in humans, it is clear that we do not completely understand the condition. As diagnostic tests and more qualitative evaluation techniques become available, we may get closer to the answers.
Evaluation of spasticity for surgical management
The surgical management of spasticity should focus on the functional evaluation of the patient. Meticulous questioning and evaluation of the patient during their daily activities and of their lifestyle is much more revealing than the most thorough neurological examination. The approach to taking the history and examining the patient should reflect this. It is invaluable to evaluate a patient in their home environment. A video recording taken by a relative of the patient sitting in a chair, lying on the bed, standing and walking is also of great benefit. This allows one to assess function in a familiar environment. Careful observation of the patient while the family, or the patient, recounts the history is often much more beneficial than a physical examination.
The following points are important to observe in the history:
• antenatal events and maternal health
• perinatal events, such as hypoxia, prolonged labour, premature rupture of the membranes, HIV exposure, Apgar scores
• family history of neurological diseases
• trauma
• previous operations for spasticity or orthopaedic deformities or corrections
• current and previous medications
• intervals of botulinum toxin injections and splinting, if any.
A detailed systemic history with specific attention to bladder and bowel habits is also important. One needs to enquire about respiratory tract infections, as patients with cerebral palsy are more prone to such infections. Throughout the examination, careful attention should be paid to determine whether the problematic spasticity is focal or diffuse, and whether there are dystonic features associated with or underlying the spasticity. The latter has significant therapeutic implications.
Physical examination of patients with spasticity should be inititated by evaluating function:
• eating or drinking, dressing and communicating while sitting
• standing upright from a seated position on own wheelchair, a chair or a bed
• balancing in standing and sitting positions
• gait with and without support or frames
• gait and standing with and without an orthosis
• ability to climb stairs
• running and jumping (if possible).
Once these functional activities have been evaluated, the focus should move towards more specific aspects of spasticity:
• Posture when lying, sitting and standing is important - note pelvic alignment and spinal deformities.
• Trunk and neck tone while sitting and standing.
• Muscle power in a functional motion, such as kneeling, sitting, squatting, and climbing a step. Traditional measures of power are not reliable in a patient with abnormal tone.
• Range of motion in all joints, especially around the hips and ankles.
• Evaluation of muscle tone, as spasticity is a velocity-dependent increase in tone. Pay attention to the difference between active and passive movements and tone.
• With regard to deep tendon reflexes, pay specific attention to crossing over of reflexes to other limbs as well as spreading of reflexes in the same limb.
• Presence of pathological reflexes, such as clonus and the Babinski sign.
• A good screening sensory examination that focuses on proprio-ception and light touch is important for future surgical interventions.
• Establish whether the spinal deformity has been corrected or is correctable.
Management of spasticity
Spasticity in itself is not a problem; its presence may be protective in preventing the patient from further injuring a limb or joint, especially in focal spasticity, which occurs after a stroke in an upper limb. The presence of spasticity may increase the functional abilities in some patients, e.g. children with spastic diplegia may be able to stand, because they may use their hip adductor spasticity to increase truncal tone, which forms a stable base for them to stay erect.
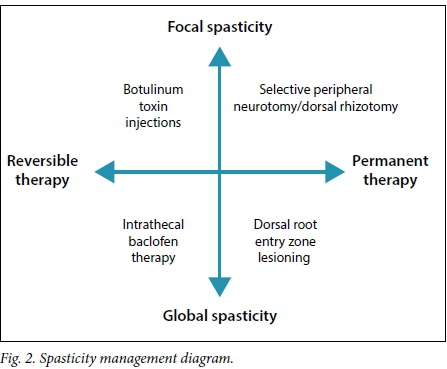
Once spasticity leads to pain and deformities or it starts to impair function, treatment to reduce it becomes important. In the evaluation of spasticity the clinician must decide whether it is focal or diffuse/ global. Fig. 2 demonstrates how this important differentiation is used to decide which therapeutic modality is potentially useful. There is, for example, no place for the use of intrathecal baclofen in patients with focal hand spasticity. It is also not possible to administer botulinum toxin injections or perform a peripheral neurotomy on every muscle involved in diffuse spasticity, such as in a child with spastic quadriplegia.
Physical and occupational therapy remain at the core of any good rehabilitation and care programme for all patients with spasticity. Speech therapists and psychology services also have an important role in the holistic care plan. The entire team must understand the functional goals of surgery, and often training that starts ahead of surgery is extremely beneficial in optimising the patient for the procedure, but also in learning new exercises that follow the surgery.
The various surgical treatments for patients with function-limiting spasticity are listed in Table 1. As a rule, most patients with spasticity should be on oral baclofen as an empiric antispasticity medication. If pain forms a major part of the spasticity, either as an exacerbation factor or owing to the spasticity, pain-modulating medications that also have an effect on spasticity, such as gabapentin, may be added.

Once these non-surgical management options have been exhausted, with no improvement in the function of the patient, surgical management may become relevant.
Selective peripheral neurotomy. The peripheral nerve is dissected free and the motor branches are mapped out with the use of a nerve stimulator. Once absence of a sensory branch of the nerve is confirmed, about 80% of the nerve to the spastic muscle is sectioned and 5 mm are resected. The goal is to restore the balance between the agonist and antagonist muscles around a joint. It is therefore only appropriate for focal spasticity around one or two joints, e.g. a hemiplegic patient after a stroke, who is starting to stand but cannot stand on a flat foot owing to inversion of the foot, resulting in pain. Performing a peripheral neurotomy on the posterior tibial nerve, targeting the branches to the tibialis posterior muscle, can allow the patient to stand on a flat foot with less pain. The muscle is usually not perceived to be weaker, as the number of motor units innervated by the remaining nerve fascicles increases significantly within a few weeks and there should not be any discernable sensory deficit after the procedure.
Baclofen. This acts as a gamma-aminobutyric acid (GABA) β-receptor agonist, leading to a reduction in calcium inflow to nerve terminals and a reduction in excitation in neurotransmitters that are released in the spinal cord.[6] While baclofen can be administered orally, the side-effects are often not tolerated and an attractive alternative is direct administration.
Intrathecal baclofen (ITB). This is provided by an implanted pump. A small catheter is placed into the thecal space (subarachnoid spinal canal), with the tip placed in the high cervical area to treat dystonia or spastic quadriplegia, or in the low thoracic area to treat spastic diplegia. Baclofen can then be administered in a slow continuous infusion at a much lower dose than oral baclofen. This leads to better bioavailability at the receptors in the spinal canal and fewer systemic effects. A key benefit of ITB is that it is reversible and very effective in patients with dystonia and spasticity.
Selective dorsal rhizotomy. This is a surgical procedure that is well proven in children with spastic diplegia and was adopted worldwide following Warwick Peacock's work in Cape Town in the 1980s. During this procedure, a multilevel laminotomy is performed from the L2 - S1 level, the cauda equina is exposed and the dorsal root is separated from the ventral root. The dorsal root is then split, typically into 3 - 4 fascicles, and guided by intraoperative neurophysiology, between 30% and 60% of the dorsal root being sectioned. With the use of this technique the monosynaptic reflex arc is interrupted, which reduces the spasticity. Key to this operation is good strength and postural stability in the child before surgery is considered. Pelvic control and strength of hip extension and abduction with good knee extension are very important in rehabilitation and improving gait in these patients.
Dorsal root entry zone lesioning (DREZotomy). During this procedure the point where the dorsal nerve roots enter the spinal cord is cauterised up to a specific depth. This interupts the interneurons for muscle tone in the Rexed laminar levels. The great benefit of this procedure is that it can be done selectively and for multiple levels, depending on the individual's need. DREZotomy is also an excellent operation for pain; with the correct technique a patient with severe pain due to uncontrolled spasticity may obtain significant relief of both.
The techniques discussed above are mostly irreversible (Fig. 2). It is therefore very important to determine what the expected functional effect of a procedure will be. Muscle tone is lower after surgery, but one also needs to have a concept of the patient's function afterwards. For example, it is not helpful to have improved leg tone but be unable to stand or walk if one could do this before the procedure. The expected effect can be tested by using botulinum toxin in selective muscles, temporarily administering ITB via an infusion pump, or performing a peripheral nerve block with a long-acting neural anaesthetic agent. Functional activity can now be evaluated with the temporary reduction in muscle tone. Reliable intraoperative neurophysiology is mandatory during surgical techniques for spasticity. These techniques are used to preserve bladder and bowel function and to guide fascicular dissection.
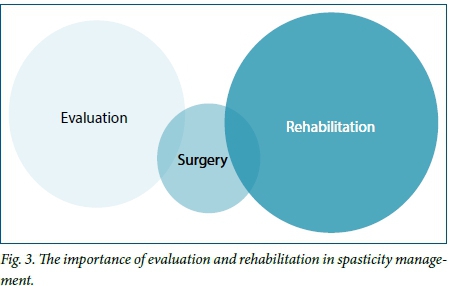
Surgery forms a small part of the entire process involved in managing patients with spasticity. As illustrated in Fig. 3, careful evaluation and appropriate surgical procedures, performed with the correct goal in mind, need to be followed by active patient and family participation in a structured rehabilitation programme that starts as soon as possible after surgery. The goals of the patient, therapist, relatives, caregivers and surgeon all need to be aligned and specific functional activities developed that will improve certain aspects of the recovery and rehabilitation process. There is no one recipe that fits all patients.
Outcomes of surgery for spasticity
Clear goals need to be discussed with the family and the management team before surgery. Expectations need to be tailored to what is achievable. It is not possible, for example, for a child who is completely dependent for all daily activities (gross motor function classification system (GMFCS) level 5) to walk independently after any form of surgical therapy. Any surgeon claiming this possibility is headed for a disaster.
The goal of surgical therapy may not necessarily be for the patient to walk independently. A realistic goal for a 65-year-old woman with left hemiplegia after a stroke may be to be able to hold a glass of water with her left hand again, or to stand with a flat foot while using a walking stick. A surgical goal in a spastic quadriplegic child who is wheelchair bound, may be for the child to be able to lie down flat on his back in bed while asleep, without hips and knees pulling up into flexion continuously. This reduction in spasticity around the hips may allow improved personal care and reduced bladder infection, as the hips can now be abducted to allow easier nappy changes and cleaning. Improving the ability for a wheelchair-bound child to sit more easily in a wheelchair with comfortable knee and hip flexion is also a great achievement in a severely spastic child who has difficulty being placed in a wheelchair owing to severe hip and knee spasticity. At the same time, it is possible for a child with spastic diplegia to walk unaided after a selective dorsal rhizotomy if he has good trunk and hip strength. Small improvements on a rating scale may lead to large functional gains in a patient.
The preoperative goals of surgery should be clearly defined by the family and the rehabilitation team. The outcome should be functional; this same function should be targeted in personalising the therapeutic strategy and the postoperative outcomes evaluation.
ITB shows very good reduction in spasticity and painful deformities caused by this condition in 80 - 97% of cases.[7] The reduction in spasticity is documented with a 2 - 3-point improvement in the 5-point Ashworth scale, and tolerance is generally very good.[7]
Peripheral neurotomies have an 80 - 85% success rate in reducing painful deformities and spasticity in focal conditions.[7] This leads to improved functional activity and prevents worsening of deformities caused by increased tone.
DREZotomy leads to a 70 - 82% decrease in spasticity, while at the same time relieving pain in paraplegic patients in up to 91% of cases.[7]
A 20-year follow-up by the Cape Town group[8] shows very good long-term outcomes in patients after selective dorsal rhizotomy. In a group of 14 patients, all but one had long-term control of their spasticity and good functional improvement that lasted into adulthood.
Complications of surgery for spasticity
As the purpose of the surgical treatment of spasticity is to improve function, possible complications must be carefully considered. It is particularly important that a wheelchair-bound patient does not have 'useless hypotonia' after surgery. Concerns about impaired bladder and bowel control, as well as spinal deformities, are also frequently raised.
Tsirikos[9] reviewed reports on the incidence of spinal deformities in the cerebral palsy population and found an incidence of 20 - 25%. Some studies report up to a 74% incidence in spastic quadriplegic patients;[10] it is important to keep this natural history in mind when considering surgical management of spasticity and its influence on spinal deformity. In the 20-year study by Langerak et al.[8] - to date the study with the longest duration - no patients experienced spinal deformity that required further surgery.
Urological dysfunction among patients with spasticity, and especially the cerebral palsy group, is much more common than previously thought. Mobility, age, communication problems and spasticity of the detrusor muscle all play a role.[10] Murphy et al.[11] have an interest in cerebral palsy patients and bladder function; 16.4% of their cohort showed spastic, hyperreflexic bladders on urodynamic testing. There were no bladder or bowel complications reported in the selective dorsal rhizotomy cohort in the Cape Town study.[8] Specific enquiry and examination, often with invasive urodynamic testing, are important in patients with cerebral palsy, as resultant recurrent urinary tract infections may lead to renal injury in the long run. Major leaps in surgical techniques, but more importantly in intraoperative neurophysiological monitoring, have allowed surgeons to reduce the incidence of all these complications.
Summary
Surgery plays an important role in the multimodal management of spasticity. While every patient with spasticity can be helped, not all require an operation. In cases where an operation may benefit the patient, it is not a one-procedure-for-all scenario. Only a team, which consists of a neurosurgeon, orthopaedic surgeon, developmental paediatrician and rehabilitation therapists, can offer each patient an objective opinion on therapeutic options. Thorough examination and multiple visits are key to planning a management strategy. The distribution of spasticity is important, as this has implications with regard to surgical management options, and every patient should be carefully screened for the presence of dystonia. Patients who do not respond well to surgical procedures for spasticity are often those in whom a coexistence of dystonia was missed. The role of the family and video analysis of the patient in their home and work environment are important in determining the patient's functional impairment. These impairments have to be incorporated in the surgical and rehabilitation goals of the patient, as individualisation of the therapeutic plan is crucial.
References
1. Lance JW. Symposium synopsis. In: Feldman RG, Young RR, Koella WP, eds. Spasticity: Disordered Control. Chicago: Yearbook Medical, 1980:485-494. [ Links ]
2. Young RR. Spasticity: A review. Neurology 1994;44:512-520. [ Links ]
3. Odding E, Roebroeck ME, Stam HJ. The epidemiology of cerebral palsy: Incidence, impairments and risk factors. Disabil Rehabil 200638(4):183-191. [ Links ]
4. Sherrington CS. Nobel lecture: Inhibition as a coordinative factor. http://www.nobelprize.org/nobel_prizes/medicine/laureates/1932/sherrington-lecture.html (accessed 29 June 2016). [ Links ]
5. Nielsen JB, Crone C, Hultborn H. The spinal pathophysiology of spasticity - from a basic science point of view. Acta Physiol (Oxf) 2007;189(2):171-180. [ Links ]
6. Scherkenbach LA, Coles LD, Patterson EE, et al. Pharmacokinetics and pharmacodynamics of intravenous baclofen in dogs: A preliminary study. J Pharm Pharmacol 2014;66(7):935-942. DOI:10.1111/jphp.12221 [ Links ]
7. Sindou M, Georgoulis G, Mertens P, eds. Neurosurgery for Spasticity: A Practical Guide for Treating Children and Adults. Vienna: Springer, 2014. [ Links ]
8. Langerak NG, Lamberts RP, Fieggen AG, et al. Selective dorsal rhizotomy: Long-term experience from Cape Town. Childs Nerv Syst 2007;23(9):1003-1006. [ Links ]
9. Tsirikos AI. Development and treatment of spinal deformity in patients with cerebral palsy. Indian J Orthop 2010;44(2):148-158. DOI:10.4103/0019-5413.62052 [ Links ]
10. Borzyskowski M. Cerebral palsy and the bladder. Dev Med Child Neurol 1989;31:687-689. [ Links ]
11. Murphy KP, Boutin SA, Ide KR. Cerebral palsy, neurogenic bladder, and outcomes of lifetime care. Dev Med Child Neurol 2012;54(10):945-950. DOI:10.1111/j.1469-8749.2012.04360 [ Links ]
 Correspondence:
Correspondence:
J M N Enslin
enslin@functionalneurosurgery.co.za














