Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.106 no.7 Pretoria 2016
http://dx.doi.org/10.7196/samj.2016.v106i7.10405
RESEARCH
How long are elderly patients followed up with mammography after the diagnosis of breast cancer? A single-centre experience in a developing country
Y ParagI; I BuccimazzaII
IMB ChB, FCRad (Diag) (SA); Department of Radiology, Faculty of Health Sciences, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
IIMB ChB, FCS (SA), FACS Breast Unit, Department of General Surgery, Faculty of Health Sciences, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
ABSTRACT
BACKGROUND: The effect of breast cancer on elderly South African (SA) patients is not well characterised. The lack of data with regard to disease burden, post-treatment surveillance and breast cancer relapse poses a challenge to providing optimum follow-up care to this group of patients.
OBJECTIVES: To assess the effect of breast cancer and adherence to post-treatment surveillance programmes among the local elderly population attending the breast oncology clinics at Addington and Inkosi Albert Luthuli Central hospitals in Durban, KwaZulu-Natal, SA.
METHODS: A retrospective review was undertaken of all patients aged >65 years diagnosed with breast cancer during 2007. Hospital records were reviewed for a period of 5 years to ascertain the stage of the disease, treatment received, adherence to post-treatment surveillance mammograms, incidence of new mammographic findings and recurrence, site of recurrence, mode of detection of recurrence, disease-free survival, and overall survival rates at 5 years.
RESULTS: In our study, the incidence of breast cancer in the elderly population was 26.7%. A significant percentage of patients (56.3%) were diagnosed at an advanced stage of disease. Of the 46.9% who had received surveillance mammography, only 6.3% received their post-treatment surveillance mammograms on time, in accordance with international recommendations. New mammographic findings were detected in 26.7% of patients during the 5-year follow-up. During the follow-up period, 15.6% of the total number of study patients presented with disease recurrence. Eighty percent of cases of recurrence were detected clinically. The overall survival at 5 years was 65.6%.
CONCLUSION: Our study highlights the significant number of elderly patients with advanced disease at diagnosis, poor compliance with internationally recommended annual post-treatment surveillance mammograms, and the relatively low overall 5-year survival rate compared with that of international studies.
According to the latest cancer statistics from the South African (SA) National Cancer Registry 2010, breast cancer is ranked as the most common cancer in women (20.62%), with an overall lifetime risk of developing breast cancer of 1 in 35 women.[1]
Increasing age remains the largest single risk factor for the development of breast cancer.[2] Despite this significant association, breast cancer survivors diagnosed at >65 years of age represent an understudied population in cancer research, with age and comorbid chronic conditions the primary reasons for their exclusion from clinical trials.[3] Conventionally, elderly has been defined as a chronological age of >65 years.[4] To date, no data exist in SA on the incidence of breast cancer in the elderly population.
Furthermore, post-treatment follow-up practices are not well known. Current surveillance programmes are adopted from the American Society of Clinical Oncology, which recommends regular clinical assessment and annual mammographic surveillance to detect disease relapse.[5] No cut-off age for following up these patients has been defined. Because of the increased risk of developing a second primary breast cancer and the risk of relapse, this screening protocol has been shown to be a cost-effective tool internationally, with adequate surveillance associated with improved survival and decreased mortality.
Despite the acknowledged rationale for annual surveillance mammograms, a study by Doubeni et al.[6]demonstrated a decline from 80.0% to 63.0% in the use of mammography among breast cancer survivors over a 5-year period, especially in older women with comorbid illnesses or those with late-stage disease.
The lack of data on the incidence, post-treatment surveillance practices and breast cancer relapse rates in the elderly SA population poses a challenge to providing optimum follow-up care for breast cancer survivors. Surveillance breast imaging regimens and intervals are adopted from international guidelines, which has implications for healthcare utilisation, cost and mortality. Knowledge of relapse-specific intervals aids in focusing surveillance at these identified periods, allows early detection of recurrence, and assists in promoting improved health awareness for our patients. Understanding relapse rates in the elderly patient also has important consequences for treatment practices, particularly in a population where morbidity from ageing and comorbid conditions assumes a greater impact on lifestyle, adherence practices and prognosis.
Objectives
The objective of our study was to assess the effect of breast cancer on elderly SA patients. To date, no research on this subject has been undertaken locally. By assessing follow-up practices and post-treatment mammographic findings, it is hoped that the currently accepted international surveillance protocols may be reviewed and, if necessary, adapted to better suit the SA experience of the disease.
Methods
A retrospective review was undertaken of all patients with histologi-cally proven breast cancer diagnosed at >65 years of age, attending the breast oncology clinics at Addington and Inkosi Albert Luthuli Central hospitals, Durban, KwaZulu-Natal, SA during the defined study time period. Patients diagnosed with breast cancer between January and December 2007 were entered into the study and followed up for a 5-year interval from January 2008 to December 2012.
Of the 150 patients diagnosed with breast cancer during 2007, 40 were >65 years of age. Of the 40 women eligible for the study, one was excluded owing to the presence of a second malignancy, diagnosed within 5 years of incident breast cancer diagnosis. A complete dataset was not obtained for a further 7 patients, who were also excluded. Thirty-two patients were included in the final analysis.
Data were extracted from patient charts retrospectively. Study numbers were used instead of patient identifiers during data collection, analysis and presentation of results to maintain patient anonymity. Information with regard to gender, age at diagnosis, stage of index breast cancer, and treatment received were recorded from the surgical breast clinic files. Patients were then followed up at the oncology clinics after treatment, where data on surveillance with annual mammograms, new mammographic findings, site of recurrence, mode of detection of recurrence, and survival outcome were recorded. Patients who had defaulted on their follow-up clinic appointments were contacted telephonically to assess survival outcome. Family members were contacted for those patients who were lost to follow-up and who could not be contacted.
Data were entered into a database supported by Excel (Microsoft, USA). The data collected were subsequently analysed using SPSS version 21 (IBM, USA). Survival analysis was recorded using the Kaplan-Meier curve.
Institutional approval was obtained from the hospitals' management and the University of KwaZulu-Natal Biomedical Research Ethics Committee (BE072/13).
Results
Of the 150 patients diagnosed with breast cancer during 2007, 40 were >65 years of age, compatible with an incidence of 26.7%. All 32 patients included in the final analysis were women, and most (43.8%) were aged between 65 and 69 years (Table 1).

A significant percentage of patients were diagnosed at an advanced stage of their disease; 3.1% were diagnosed with stage 1, 28.1% with stage 2, 31.3% with stage 3, and 25.0% with stage 4 disease, while 12.5 % were incompletely staged (Table 2).

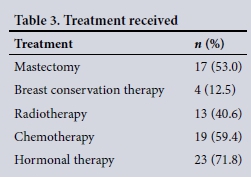
A total mastectomy was performed in 53.0% of patients and 12.5% were treated with breast conservation therapy. Radiotherapy was administered to 40.6% of patients, chemotherapy to 59.4%, and hormonal therapy to 71.8% (Table 3).
Fifteen patients were followed up with surveillance mammograms. Only 6.3% (2 of 32) of patients received their post-treatment surveillance mammograms on time annually for 5 years. The remaining 40.6% (13 of 32) had mammographic surveillance at some stage during the 5-year follow-up period; not annually, however, as per international recommendations. The number of mammograms performed in this group ranged from 1 to 4 over 5 years. With regard to mammographic surveillance, 18.8% (6 of 32) were non-compliant, with no mammograms being performed during the 5-year follow-up period, while 34.4% (11 of 32) were excluded, either owing to bilateral mastectomies or stage 4 disease at presentation.
Of the 15 patients who were followed up with mammograms, either on time or delayed, 26.7% (4 of 15) had new mammographic findings during the 5-year follow-up period. Of these, 1 patient had benign radiological findings, and 3 had malignant disease (either radiologically suspected or biopsy proven), ranging from locoregional recurrence (2 of 15) to a new second malignancy not related to breast cancer (1 of 15).
Disease recurrence occurred in 15.6% (5 of 32) of the total number of patients studied during the 5-year follow-up period. Of these 5 patients, 3 presented with distant metastases, 1 with locoregional recurrence, and 1 with both locoregional recurrence and distant metastases detected on the same follow-up date. With regard to mode of detection of recurrence, 80.0% (4 of 5) were detected clinically and 20.0% (1 of 5) radiologically.
Over the 5-year follow-up, 34.4% of patients remained disease free, 34.4% died, 15.6% were lost to follow-up, and 15.6% were classified as 'other'. The 'other' category included patients with known metastases at the time of diagnosis, who had stable disease after 5 years of follow-up (but who were not disease free), or those who were seen in the clinic but defaulted on their mammograms and could not be contacted after 5 years. According to the Kaplan-Meier curve, the overall survival at 5 years was 65.6%.
Discussion
Cancer is an emerging health problem in SA. The latest cancer statistics from the SA National Cancer Registry 2010, rank breast cancer as the most common cancer in women (20.6%), with an overall lifetime risk of developing the disease of 1 in 35.[1]
According to the mid-year statistics released by Statistics SA in July 2015, ~8.0% (4 million) of SA's estimated population of almost 55 million are documented to be >60 years of age. Of this older population, ~51.0% are female. Life expectancy at birth for 2015 was estimated at 60.6 years for males and 64.3 years for females, which has increased from the 2011 statistics of 54.9 years and 59.1 years for males and females, respectively.[7] The proportion of elderly persons aged >60 years is therefore increasing over time, similar to worldwide trends of rising life expectancy.[8]
No previous data exist on the incidence of breast cancer in the elderly SA population. In our study, the incidence of breast cancer in the elderly was 26.7%, just over a quarter of all patients with newly diagnosed breast cancer during 2007. In the USA, with an estimated total population of 318.9 million (2014), >40.0% of new breast cancer cases are diagnosed in women >65 years of age.[9] As breast cancer incidence increases with age, worldwide changing demographics and increasing life expectancy are anticipated to further increase the absolute number of older women with breast cancer.[8]
Increasing age remains the largest single risk factor for the development of breast cancer, with ~50.0% of breast cancer cases occurring in women >65 years and >30.0% in women >70 years.[2] Our study demonstrated similar findings, with 43.8% of patients diagnosed at 65 - 69 years, and 44.0% at >75 years.
A significant percentage of patients in our study were diagnosed at an advanced stage of disease, with more than half (56.3%) presenting with stage 3 and 4 disease. A total of 31.2% of patients presented with stage 1 and 2 disease. Possible reasons include lack of healthcare information, lack of adequate screening programmes, socioeconomic factors, distance from healthcare centres, effects of comorbid conditions, and decreased mobility resulting in delay in seeking medical attention. This advanced stage of disease also impacted on treatment practices, with only 65.5% of patients receiving surgical management for primary breast cancer. Studies have shown that older patients are not treated to the same extent as younger patients, and increasing age at diagnosis predicts deviation from guidelines for all treatment modalities. Evidence-based medicine in older patients is lacking, as they are often excluded from clinical trials because of existing comorbidities and limited life expectancy.[8] Comparative studies with a younger cohort are needed to further define treatment practices between the two age groups in our setting.
The latest practice guidelines from the American Society of Clinical Oncology recommend regular history-taking, physical examination, and yearly mammography for breast cancer follow-up.[5] No cut-off age for following up these patients has been defined. Yearly mammographic surveillance of women who have undergone curative treatment has been shown to be highly cost-effective because these patients are at an increased risk of developing a second primary breast cancer. Furthermore, several studies have demonstrated that such mammographic surveillance is associated with improved survival and a decreased breast cancer mortality rate in older patients with early-stage disease.[10]
Our study demonstrated poor compliance with the recommended international guidelines. Only 6.3% of patients were followed up with yearly mammograms, while in a significant percentage (40.6%) mammography had been delayed. Some of the reasons attributable for the delay were lack of referral, non-compliance with follow-up appointments, and limited resources.
Despite this poor compliance, new mammographic findings were detected in a noteworthy 26.7% of patients who underwent mam-mography at some stage during the 5-year period. Of these patients, 20.0% had findings of malignancy.
International figures suggest that about 30.0% of women develop recurrence after treatment for primary breast cancer, figures for early stage disease being lower.[11] The risk of new or recurrent breast cancer is increased among women with known disease compared with those without a history of breast cancer. Karam[12] noted that the majority of breast cancer recurrences occur during the first decade after initial diagnosis, with a peak incidence 2 - 5 years after diagnosis. Surveillance is therefore emphasised most during the initial 5 years after therapy, when the risk of relapse is at its highest. Bosco et al.[13] concluded that although recurrence occurs in relatively few older women after 5 years, those in whom it does occur often have advanced cancers that are difficult to treat. To improve outcomes for these older women, particularly those with a >5-year life expectancy, surveillance for recurrence after 5 years of survival is essential to reduce morbidity and mortality and to maintain quality of life.
Our study demonstrated a 5-year recurrence rate of 15.6%, which is lower than international figures of ~30.0%. However, our figures are to be viewed with caution owing to the number of patients lost to follow-up during the study. The site of recurrence, however, was comparable with that of international studies, with distant metastases being more commonly detected than locoregional recurrence in patients presenting with relapse. With regard to mode of detection of recurrence, the majority were detected clinically rather than radiologically during surveillance periods, similar to findings of international studies.
Our study demonstrated that only 34.4% of patients remained disease free after 5 years. The remainder of patients were alive with metastatic disease, were lost to follow-up, or had died.
The overall survival at 5 years was 65.6%. This figure is significantly lower than international figures obtained from the American Cancer Society and National Cancer Institute Surveillance Epidemiology and End Results (SEER) database, which reported a 5-year survival rate for breast cancer of 89.0%, a 10-year rate of 83.0%, and a 15-year rate of 78.0% for all stages of disease combined.[14] Our lower figures were influenced by the number of patients who were lost to follow-up (15.6%) and the significant number of patients who died (34.4%) during the follow-up period. Owing to poor patient records, the cause of death could not be determined, i.e. related to breast cancer or caused by patient comorbidity. Late stage of disease at diagnosis with poor follow-up practices are thought to be critical factors affecting the overall survival in our setting.
Study limitations
Limitations of the study include the retrospective study design and the inclusion of a single centre. A further limitation is the lack of comparison with a younger cohort, thus not permitting any firm conclusions or recommendations to be made. This study will, however, provide the stimulus for future larger and more comparative studies that specifically look at outcomes and follow-up after completing primary breast cancer treatment. Poor patient follow-up, lack of adequate patient records, and lack of updated cancer registries indicating patient outcomes are further limitations.
Conclusion
Our study highlights poor compliance with internationally recommended annual post-treatment mammograms in elderly patients diagnosed with breast cancer. Further studies with younger cohorts are necessary to ascertain whether this is a universal problem or unique to the older cohort. In patients who underwent follow-up mammograms, just over a quarter presented with new mammographic findings - the majority of these cases with features of malignancy. Health promotion and education are essential components of reducing the number of patients presenting with advanced disease, which impacts on treatment practices and overall survival. Further education of healthcare providers with regard to breast cancer screening and follow-up regimens is necessary to allow early diagnosis, standardised care, optimised referrals and more efficient detection of recurrence. Larger studies with follow-up over longer periods are required to more definitively assess relapse rates and relapse-specific intervals, with the objective of tailoring post-treatment surveillance programmes to better suit a resource-limited setting.
References
1. South African National Cancer Registry, 2010. http://www.nioh.ac.za (accessed 20 May 2016). [ Links ]
2. Bernardi D, Errante D, Galligioni E, et al. Treatment of breast cancer in older women. Acta Oncol2008;47(2):187-198. DOI:10.1080/02841860701630234 [ Links ]
3. Patnaik JL, ByersT, Diguiseppi C, et al. The influence of comorbidities on overall survival among older women diagnosed with breast cancer. J Natl Cancer Inst 2011;103(14):1101-1111. DOI:10.1093/jnci/djr188 [ Links ]
4. Orimo H, Ito H, Suzuki T, et al. Reviewing the definition of 'elderly. Geriatr Gerontol Int 2006;6:149- 158. DOI:10.1111/j. 1447-0594.2006.00341.x [ Links ]
5. Khatcheressian JL, Hurley P, Bantug E, et al. Breast cancer follow-up and management after primary treatment: American Society Of Clinical Oncology clinical practice guideline update. J Clin Oncol2013;31(7):961-965. DOI:10.1200/JCO.2012.45.9859 [ Links ]
6. Doubeni CA, Field TS, Yood MU, et al. Patterns and predictors of mammography utilization among breast cancer survivors. Cancer 2006;106(11):2482-2488. DOI:10.1002/cncr.21893 [ Links ]
7. Statistics South Africa. Mid-year population estimates, 2015. http://www.statssa.gov.za/publications/2015 (accessed 20 May 2016). [ Links ]
8. Markopoulos C, van de Water W. Older patients with breast cancer: Is there bias in the treatment they receive? Ther Adv Med Oncol 2012;4(6):321-327. DOI:10.1177/1758834012455684 [ Links ]
9. National Cancer Institute. Surveillance Epidemiology, and End Results Program, 2011. http://seer.cancer.gov (accessed 20 May 2016). [ Links ]
10. Margenthaler J, Allam E, Chen L, et al. Surveillance of patients with breast cancer after curative-intent primary treatment: Current practice patterns. J Oncol Pract 2012;8(2):79-83. DOI:10.1200/JOP.2011.000289 [ Links ]
11. Hiramanek N. Breast cancer recurrence: Follow up after treatment for primary breast cancer. Postgrad Med J 2004;80:172-176. DOI:10.1136/pgmj.2003.010728 [ Links ]
12. Karam A. Breast cancer posttreatment surveillance: Diagnosis and management of recurrent disease. Clin Obstet Gynaecol 2011;54(1):157-163. DOI:10.1097/GRF.0b013e318208393b [ Links ]
13. Bosco JL, Lash TL, Prout MN, et al. Breast cancer recurrence in older women five to ten years after diagnosis. Cancer Epidemiol Biomarkers Prev 2009;18(11):2979-2983. DOI:10.1158/1055-9965.EPI-09-0607 [ Links ]
14. Cancer.Net Breast cancer - statistics, 2015. http://www.cancer.net/cancer-types/breast-cancer/statistics/2015 (accessed 20 May 2016). [ Links ]
 Correspondence:
Correspondence:
Y Parag
yethiksha@gmail.com
Accepted 18 March 2016.














