Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.106 no.5 Pretoria Mai. 2016
http://dx.doi.org/10.7196/samj.2016.v106i5.10269
RESEARCH
National priorities for perioperative research in South Africa
B M BiccardI, II; C S AlphonsusIII; D G BishopIII; L CronjeIII; H-L KluytsIV; B KuselV; S MaswimeVI; R OoditVII; A R ReedVIII; A M TorborgIX; R WiseX; on behalf of the South African Perioperative Research Group national research priority-setting working group
IPhD;Department of Anaesthesia and Perioperative Medicine, Groote Schuur Hospital and Faculty of Health Sciences, University of Cape Town, South Africa
IIPhD; Perioperative Research Group, Discipline of Anaesthesiology and Critical Care, School of Clinical Medicine, College of Health Sciences, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
IIIFCA (SA) ; Perioperative Research Group, Discipline of Anaesthesiology and Critical Care, School of Clinical Medicine, College of Health Sciences, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
IVMMed (Anaes) Department of Anaesthesiology, School of Medicine, Faculty of Health Sciences, University of Pretoria, South Africa
VFCA (SA), MMed;Perioperative Research Group, Discipline of Anaesthesiology and Critical Care, School of Clinical Medicine, College of Health Sciences, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
VIFCOG (SA), MMed; Department of Obstetrics and Gynaecology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
VIIFCS (SA); Discipline of General Surgery, Faculty of Health Sciences, University of Cape Town and Groote Schuur Hospital, Cape Town, South Africa, and Matley and Partners, Cape Town
VIIIFRCA, MMed (Anaes); Department of Anaesthesia and Perioperative Medicine, Groote Schuur Hospital and Faculty of Health Sciences, University of Cape Town, South Africa
IXFCA (SA); Perioperative Research Group, Discipline of Anaesthesiology and Critical Care, School of Clinical Medicine, College of Health Sciences, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
XFCA (SA), MMed (Anaes), Cert Crit Care (SA); Perioperative Research Group, Discipline of Anaesthesiology and Critical Care, School of Clinical Medicine, College of Health Sciences, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
ABSTRACT
BACKGROUND. Perioperative research is currently unco-ordinated in South Africa (SA), with no clear research agenda.
OBJECTIVE. To determine the top ten national research priorities for perioperative research in SA.
METHODS. A Delphi technique was used to establish consensus on the top ten research priorities.
RESULTS. The top ten research priorities were as follows: (i) establishment of a national database of (a) critical care outcomes, and (b) critical care resources; (ii) a randomised controlled trial of preoperative B-type natriuretic peptide-guided medical therapy to decrease major adverse cardiac events following non-cardiac surgery; (iii) a national prospective observational study of the outcomes associated with paediatric surgical cases; (iv) a national observational study of maternal and fetal outcomes following operative delivery in SA; (v) a stepped-wedge trial of an enhanced recovery after surgery programme for (a) surgery, (b) obstetrics, (c) emergency surgery, and (d) trauma surgery; (vi) a stepped-wedge trial of a surgical safety checklist on patient outcomes in SA; (vii) a prospective observational study of perioperative outcomes after surgery in district general hospitals in SA; (viii) short-course interventions to improve anaesthetic skills in rural doctors; (ix) studies of the efficacy of simulation training to improve (a) patient outcomes, (b) team dynamics, and (c) leadership; and (x) development and validation of a risk stratification tool for SA surgery based on the South African Surgical Outcomes Study (SASOS) data.
CONCLUSIONS. These research priorities provide the structure for an intermediate-term research agenda.
Perioperative research is currently unco-ordinated in South Africa (SA). A large group of investigators and interested individuals collaborated under the auspices of the South African Perioperative Research Group (SAPORG), the members of which are listed in Appendix 1, to address this limitation. This initiative was undertaken because the group believed that: (i) collaborative research is necessary to address the clinical challenges encountered in perioperative care and outcomes, both in SA and globally; (ii) we have the capacity to conduct national and international collaborative research in SA;[1] (iii) collaborative research conserves the limited research resources in SA and globally;[2](iv) there are urgent public health issues in perioperative medicine that need to be addressed to improve the health of the SA and/or global surgical populations;[1] and (v) a national research priority-setting process[3] is necessary to prioritise research in an environment of limited research resources.
Objective
To determine the top ten national research priorities for perioperative research in SA, using a national research priority-setting process.
Methods
A Delphi technique[4] was followed for this national research priority-setting project. It was conducted in four rounds. In the first round, an open email invitation was sent to approximately 600 individuals across SA, based predominantly on South African Surgical Outcomes Study (SASOS) participation[1] together with other perioperative and critical care research leaders who were identified to the group. Furthermore, the recipients were encouraged to forward the email to other individuals who might be interested in the research-setting process. In the first round respondents were asked to submit potential research questions or research priority areas. The responses were collated, and where appropriate research questions were amalgamated by BMB. This resulted in 116 potential research priority questions, covering a broad range of questions and including proposals within the following fields: national pragmatic trials, perioperative outcomes, cardiovascular, critical care, education, obstetrics, paediatrics, trauma and resuscitation, perioperative ultrasonography, burns and perioperative airway management.
In the second round, these 116 potential research priority questions were circulated to all the respondents. They were asked to rank the top ten research questions, and where possible to provide justifications for inclusion or exclusion of priorities. In the third round, the same 116 questions were presented in rank order based on the responses from round two, with all submitted justifications attached. In this round the respondents were asked to consider re-ranking their previous submission from round two, based on the priorities ranking and justifications of the group. If the respondents preferred not to change their previous rankings, they were encouraged to provide justifications for taking such a position. For the second and third rounds of the Delphi process, the respondents were encouraged not to discuss their submissions with other colleagues to minimise bias.
The final Delphi stage took place at a workshop on 12 September 2015. At the workshop, a discussion of the justifications of the suggested top 15 research priorities was held. Following the open discussion, a final round of the Delphi process was conducted. Eight random small groups discussed and then submitted their top ten priorities from the list. The final top ten national research priorities were determined from this process.
Statistical analysis
The rank-order of the research priorities for each round was established using a reverse scoring system, i.e. a respondent's rank of 1 received 10 points, down to a rank of 10, which received 1 point. The scores of the respondents were combined for each round to develop the research priority rank order.
Results
The top ten national research priorities for perioperative research in SA following the four rounds of the Delphi process are shown in Table 1.
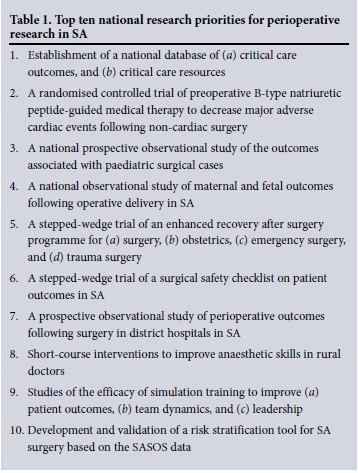
Discussion
Ten national research priorities for perioperative research in SA have been identified. These priorities provide the structure for a national collaborative perioperative research programme for the next few years in SA. Importantly, the research priorities cover a diverse field, suggesting that the process was not biased towards a single interest group. The Delphi process for research priority setting is well established, and is used in the UK to determine research priorities for potential funders.[3]
Priority 1. Establishment of a national database of (a) critical care outcomes, and (b) critical care resources
Critical care medicine crosses virtually every perioperative discipline. It is well placed to serve as a platform for perioperative research, optimisation of practices, and assessment of needs and resource allocation. However, currently these objectives cannot be met because critical care data are either not being collected in a useful format or not being shared at a national level. Many developed countries have implemented expensive databases for these purposes, but they are currently not feasible in SA because of financial, resource or manpower constraints.[5-7]
As critical care is an over-subscribed resource in SA,[1] a national critical care database would allow for the monitoring of the success of national interventions to improve outcomes, quality performance evaluation, and future planning and management of resources.[8] The SAPORG aims to create a critical care platform through integration of a minimum dataset from current regional databases. In addition, critical care databases currently in use that are deemed suitable for national use will be offered for use around the country. The goal is to establish a national database system that is of low cost, flexible for the needs of research and clinical use, and integrated into critical practice in order to negate the duplication of data capture for both research and clinical work.
Priority 2. A randomised controlled trial of preoperative B-type natriuretic peptide (BNP)-guided medical therapy to decrease major adverse cardiac events following non-cardiac surgery
Integration of biomarkers into clinical practice is based on a progressive six-phase evaluation, which includes: (i) proof of concept; (ii) prospective validation; (iii) demonstration of incremental value; (iv) clinical utility; (v) improved clinical outcome; and (vi) cost-effectiveness.[9] The current data on BNP for preoperative risk assessment fulfil proof of concept for cardiovascular compli-cations,[10,11] validation in prospective studies,[12,13] incremental value by significantly improving the revised cardiac risk index's prediction of major adverse cardiac events,[14] and clinical utility through a significant improvement in preoperative risk classification.[15] Importantly, the fifth stage of integration of a biomarker into clinical practice demands demonstration that biomarker-directed therapy improves clinical outcome. This has been shown in a non-surgical population[16] where a more aggressive heart failure therapy regimen driven by targeting the BNP level was generally well tolerated in the elderly and associated with significantly fewer cardiovascular events.
We believe that this is one area where as South Africans we could make a global impact on perioperative cardiovascular patient outcomes, because this is a simple intervention that is potentially readily accessible to our patients owing to bedside test availability.
Priority 3. A national prospective observational study of the outcomes associated with paediatric surgical cases
Children represent a significant proportion of the SA population, with 30% of the population aged <15 years,[17] yet there are few data on paediatric morbidity and mortality following surgery in SA. We have little information on the number of children undergoing surgery, who is providing their anaesthesia (specialist v. non-specialist anaesthetists), or the quality of the perioperative care they receive. These data are essential in order to understand the current paediatric perioperative morbidity in SA.
Performing a South African Paediatric Surgical Outcomes Study would address some of the limitations in our understanding of paediatric surgical outcomes in SA. This study would identify risk factors associated with poor outcomes, and potential interventions that could improve the quality of care and outcomes in paediatric surgery in SA in the future. These data would be valuable to clinicians, policymakers and healthcare funders.
Priority 4. A national observational study of maternal and fetal outcomes following operative delivery in SA
Perioperative maternal and fetal morbidity and mortality remain a significant public health problem in SA. The caesarean section rate has increased globally,[18] yet there remains no clear evidence that it is associated with an improvement in maternal or fetal outcomes.[19] In SA, a woman is three times more likely to die following an operative as opposed to a vaginal delivery.[20] Maternal haemorrhage is the leading cause of obstetric mortality in SA, and it accounts for a third of all deaths at caesarean section.[21] Furthermore, caesarean section is associated with increased morbidity for both mother and fetus.[22] Unfortunately there are still few data describing the determinants of adverse maternal and fetal outcomes in SA, and these data remain largely retrospective and incomplete.[21,23] There is an urgent need to evaluate maternal and fetal outcomes of operative delivery in SA.[20] Only once we understand the degree of this problem will we be able to target resource allocation and public health interventions that will improve maternal and fetal outcomes.
Priority 5. A stepped-wedge trial of an enhanced recovery after surgery (ERAS) programme for (a) surgery, (b) obstetrics, (c) emergency surgery, and (d) trauma surgery
Surgery is associated with significant morbidity and mortality. Perioperative care in SA is fragmented, with little interaction between various disciplines and role-players. Many aspects of perioperative care in SA are not evidence based, and outcome data are lacking. The ERAS programme addresses these shortcomings. It is a patient-centred, evidence-based, multidisciplinary team approach to perioperative care with clinical outcome, functional recovery, patient experience and compliance with guidelines measured and analysed. Feedback on individual and institutional performance by the profession for the profession is provided regularly.[24,25]
The programme was established by the ERAS Society and is fully integrated into perioperative care programmes in the UK, Sweden, Denmark, Spain, New Zealand and parts of Canada. It has resulted in a significant reduction in postoperative complications (40 - 50%) and length of stay (30 - 40%), and in significant cost saving.[24-27] The programme has not been implemented in a low- to middle-income country (LMIC) where resources are limited, healthcare systems differ from those in high-income countries and access to healthcare is difficult. However, the potential benefits to patients, healthcare providers and funders are potentially considerable in SA.
Priority 6. A stepped-wedge trial of a surgical safety checklist (SSC) on patient outcomes in SA
Meta-analyses of observational data have shown that the use of an SSC significantly improves patient outcomes after surgery, including a reduction in mortality.[28,29] Although there are few randomised controlled trials, they seem to support this conclusion.[30-32] Despite this compelling evidence, and health policies mandating the use of an SSC in many countries, including SA, successful uptake and implementation has not always been achieved[33] Further studies on the implementation and outcomes of the SSC would require a stepped-wedge cluster design, as it would no longer be deemed ethical to withhold the use of an SSC in a control group.[31,34] The rationale for a trial on the implementation of an SSC in SA is twofold, as the trial: (i) effects the implementation of a mandated, essential public health intervention provision; while (ii) simultaneously providing additional high-level evidence to aid universal uptake of the SSC.
Priority 7. A prospective observational study of perioperative outcomes following surgery in district hospitals in SA
Apart from the outcomes gathered by the Confidential Enquiries into Maternal Deaths,[21] relating largely to caesarean sections, there are no reliable or comprehensive data on the outcomes, or indeed on the quantity and types, of procedures that are performed at district hospitals in SA. Indeed, the SASOS had a small and unrepresentative sample of eight district hospitals in SA, constituting only 6.4% of the patients in the study.[1] Surgery is considered particularly cost-effective in LMICs,[35] even extending to the management of chronic diseases such as diabetes mellitus.[36] In order to realise these benefits at district hospital level, a prospective observational study of numbers and types of procedures, and the associated patient outcomes, will be useful to both health system managers and planners, and educators responsible for the training of future doctors in district hospitals.
Priority 8. Short-course interventions to improve anaesthetic skills in rural doctors
Anaesthesia-related maternal mortality in rural hospitals remains unacceptably high in SA.[21] Improving the anaesthetic skills of doctors practising in rural hospitals has the potential to address this important public health problem. Short-course interventions include the development of online anaesthesia courses, simulation courses and telephonic support following onsite learning. In order to assess the efficacy of these interventions, it is planned that pre- and post-course performance and subsequent retention of skills will be assessed.
Priority 9. Studies of the efficacy of simulation training to improve (a) patient outcomes, (b) team dynamics, and (c) leadership
Simulation can be used as an adjunct for training and assessment of doctors at various levels of experience. This can include new skills and emergency simulation training. Its use has become widespread in medical education, and a recent SA anaesthesia study has shown that it is feasible as an assessment tool in this country.[37] Implementing anaesthesia simulation training in SA has the potential to improve anaesthesia skills of interns, community service doctors and rural doctors.
The goal is to ensure collaboration between the current SA simulation centres in order to develop a medical simulation curriculum. A focus of the research will also be to establish acceptable patient outcomes to monitor the utility of the simulation training in SA.
Priority 10. Development and validation of a risk stratification tool for SA surgery based on the SASOS data
Risk stratification tools serve to identify characteristics that can be used to categorise patients at high risk for particular outcomes[38] and may therefore serve as prognostic tools. Risk stratification tools may further assist in identifying modifiable risk factors that can be managed to decrease the incidence of postoperative complications and mortality. Such tools can also be developed to identify interventions to improve quality of care or reduce costs. The SASOS recorded information regarding postoperative mortality, intensive care admission and duration of hospital stay in elective, urgent and emergency non-cardiac, non-obstetric cases in adults.[1] Currently this remains the only available prospective observational dataset for perioperative care in SA.
The SASOS dataset fulfils a number of the criteria necessary to develop an appropriate valid risk stratification tool for perioperative outcomes in SA, as the data are from SA surgical patients, and the tool will be applied to the same group of patients. Furthermore, the definitions for risk factors and outcomes will be consistent between the derivation and validation of the risk prediction tool.[39] It is possible that the development and validation of an SA perioperative risk stratification tool could contribute substantially to perioperative care in SA, in both the private and public sectors, through identification of risk and allocation of timeous interventions to modify perioperative risk.
Limitations of the national research priority-setting process
This is the first time such an initiative has been undertaken, and it is likely that some important role-players in perioperative research were not included in the process. However, the fact that the priorities cover a broad range of topics, and that over 600 perioperative investigators were contacted around the country, suggests that this is at least an acceptable starting point.
This process also did not include patients in the research priority-setting process. Ideally, this should be an objective for future national research priority-setting meetings.[3]
Conclusions
The top ten national priorities for perioperative research in SA are presented following a national research priority-setting process using the Delphi technique. These research priorities provide the structure for an intermediate-term research agenda.
Funding disclosure. The workshop was funded by the Discipline of Anaesthesiology and Critical Care, University of KwaZulu-Natal. It had no role in the study design, data acquisition, data analysis or writing of the article.
References
1. Biccard BM, Madiba TE. The South African Surgical Outcomes Study: A 7-day prospective observational cohort study. S Afr Med J 2015;105(6):465-475. DOI:10.7196/SAMJ.9435 [ Links ]
2. Ioannidis JP. How to make more published research true. PLoS Med 2014;11(10):e1001747. DOI:10.1371/journal.pmed.1001747 [ Links ]
3. James Lind Alliance. Priority Setting Partnerships. http://www.jla.nihr.ac.uk/ (accessed 15 October 2015). [ Links ]
4. Hsu C-C, Sandford BA. The Delphi Technique: Making sense of consensus. Pract Assess Res Eval 2007;12(10):1-8. [ Links ]
5. Harrison DA, Brady AR, Rowan K. Case mix, outcome and length of stay for admissions to adult, general critical care units in England, Wales and Northern Ireland: The Intensive Care National Audit & Research Centre Case Mix Programme Database. Crit Care 2004;8(2):R99-R111. DOI:10.1186/cc2834 [ Links ]
6. Stow PJ, Hart GK, Higlett T, et al. Development and implementation of a high-quality clinical database: The Australian and New Zealand Intensive Care Society Adult Patient Database. J Crit Care 2006;21(2):133-141. DOI:10.1016/j.jcrc.2005.11.010 [ Links ]
7. Mehmood A, Razzak JA, Kabir S, Mackenzie EJ, Hyder AA. Development and pilot implementation of a locally developed Trauma Registry: Lessons learnt in a low-income country. BMC Emerg Med 2013;13:4. DOI:10.1186/1471-227X-13-4 [ Links ]
8. Gordon K, Allorto N, Wise R. Analysis of referrals and triage patterns in a South African metropolitan adult intensive care service. S Afr Med J 2015;105(6):491-495. DOI:10.7196/SAMJ.9007 [ Links ]
9. Hlatky MA, Greenland P, Arnett DK, et al. Criteria for evaluation of novel markers of cardiovascular risk: A scientific statement from the American Heart Association. Circulation 2009;119(17):2408-2416. DOI:10.1161/CIRCULATIONAHA.109.192278 [ Links ]
10. Rodseth RN. B type natriuretic peptide - a diagnostic breakthrough in peri-operative cardiac risk assessment? Anaesthesia 2009;64(2):165-178. DOI:10.1111/j.1365-2044.2008.05689.x [ Links ]
11. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012;60(16):1581-1598. DOI: 10.1016/j.jacc.2012.08.001 [ Links ]
12. Rodseth RN, Padayachee L, Biccard BM. A meta-analysis of the utility of pre-operative brain natriuretic peptide in predicting early and intermediate-term mortality and major adverse cardiac events in vascular surgical patients. Anaesthesia 2008;63(11):1226-1233. DOI:10.1111/j.1365-2044.2008.05574.x [ Links ]
13. Karthikeyan G, Moncur RA, Levine O, et al. Is a pre-operative brain natriuretic peptide or N-terminal pro-B-type natriuretic peptide measurement an independent predictor of adverse cardiovascular outcomes within 30 days of noncardiac surgery? A systematic review and meta-analysis of observational studies. J Am Coll Cardiol 2009;54(17):1599-1606. DOI:10.1016/j.jacc.2009.06.028 [ Links ]
14. Choi JH, Cho DK, Song YB, et al. Preoperative NT-proBNP and CRP predict perioperative major cardiovascular events in noncardiac surgery. Heart 2010;96(1):56-62. DOI:10.1136/hrt.2009.181388 [ Links ]
15. Rodseth RN, Biccard BM, le Manach Y, et al The prognostic value of preoperative and postoperative B-type natriuretic peptides (BNP and NT proBNP) in patients having noncardiac surgery: A systematic review and individual patient data meta-analysis. J Am Coll Cardiol 2014:63(2):170-180. DOI:10.1016/j.jacc.2013.08.1630 [ Links ]
16. Gaggin HK, Mohammed AA, Bhardwaj A, et al Heart failure outcomes and benefits of NT-proBNP-guided management in the elderly: Results from the prospective, randomized ProBNP outpatient tailored chronic heart failure therapy (PROTECT) study. J Card Fail 2012;18(8):626-634. DOI:10.1016/j.cardfail.2012.05.005 [ Links ]
17. Statistics South Africa. Statistical release P0302. Mid-year population estimates. 2013. http://www.statssa.gov.za/publications/p0302/p03022013.pdf (accessed 14 October 2014). [ Links ]
18. World Health Organization. Statement on Caesarean Section Rates. Geneva: WHO, 2015. [ Links ]
19. Betran AP, Vindevoghel N, Souza JP, Gulmezoglu AM, Torloni MR. A systematic review of the Robson classification for caesarean section: What works, doesn't work and how to improve it. PLoS One 2014;9(6):e97769. DOI:10.1371/journal.pone.0097769 [ Links ]
20. Gebhardt GS, Fawcus S, Moodley J, Farina Z, National Committee for Confidential Enquiries into Maternal Deaths in South Africa. Maternal death and caesarean section in South Africa: Results from the 2011-2013 Saving Mothers Report of the National Committee for Confidential Enquiries into Maternal Deaths. S Afr Med J 2015;105(4):287-291. DOI:10.7196/SAMJ.9351 [ Links ]
21. Department of Health, RSA. Saving Mothers 2011-2013: Sixth report on confidential enquiries into maternal deaths in South Africa. Pretoria: Department of Health, 2015. [ Links ]
22. Villar J, Valladares E, Wojdyla D, et al Caesarean delivery rates and pregnancy outcomes: The 2005 WHO global survey on maternal and perinatal health in Latin America. Lancet 2006;367(9525):1819-1829. DOI:10.1016/S0140-6736(06)68704-7 [ Links ]
23. Patttinson R, Rhoda N. Saving Babies 2012-2013: Ninth Report on Perinatal Care in South Africa. Pretoria: Tshepesa Press, 2014. [ Links ]
24. Gustafsson UO, Scott MJ, Schwenk W, et al Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations. World J Surg 013;37(2):259-284. DOI:10.1007/s00268-012-1772-0 [ Links ]
25. Varadhan KK, Neal KR, Dejong CH, Fearon KC, Ljungqvist O, Lobo DN. The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: A meta-analysis of randomized controlled trials. Clin Nutr 2010;29(4):434-440. DOI:10.1016/j.clnu.2010.01.004 [ Links ]
26. Sammour T, Zargar-Shoshtari K, Bhat A, Kahokehr A, Hill AG. A programme of Enhanced Recovery After Surgery (ERAS) is a cost-effective intervention in elective colonic surgery. N Z Med J 2010;123(1319):61-70. [ Links ]
27. Verheijen PM, van der Ven AW, Davids PH, van der Wall BJ, Pronk A. Feasibility of enhanced recovery programme in various patient groups. Int J Colorectal Dis 2012;27(4):507-511. DOI:10.1007/s00384-011-1336-z [ Links ]
28. Bergs J, Hellings J, Cleemput I, et al. Systematic review and meta-analysis of the effect of the World Health Organization surgical safety checklist on postoperative complications. Br J Surg 2014;101(3):150-158. DOI:10.1002/bjs.9381 [ Links ]
29. Gillespie BM, Chaboyer W, Thalib L, John M, Fairweather N, Slater K. Effect of using a safety checklist on patient complications after surgery: A systematic review and meta-analysis. Anesthesiology 2014;120(6):1380-1389. DOI:10.1097/ALN.0000000000000232 [ Links ]
30. Calland JF, Turrentine FE, Guerlain S, et al. The surgical safety checklist: Lessons learned during implementation. Am Surg 2011;77(9):1131-1137. [ Links ]
31. Haugen AS, Softeland E, Almeland SK, et al. Effect of the World Health Organization checklist on patient outcomes: A stepped wedge cluster randomized controlled trial. Ann Surg 2015;261(5):821-828. DOI:10.1097/SLA.0000000000000716 [ Links ]
32. Chaudhary N, Varma V, Kapoor S, Mehta N, Kumaran V, Nundy S. Implementation of a surgical safety checklist and postoperative outcomes: A prospective randomized controlled study. J Gastrointest Surg 2015;19(5):935-942. DOI:10.1007/s11605-015-2772-9 [ Links ]
33. Pickering SP, Robertson ER, Griffin D, et al. Compliance and use of the World Health Organization checklist in UK operating theatres. Br J Surg 2013;100(12):1664-1670. DOI:10.1002/bjs.9305 [ Links ]
34. Hemming K, Haines TP, Chilton PJ, Girling AJ, Lilford RJ. The stepped wedge cluster randomised trial: Rationale, design, analysis, and reporting. BMJ 2015;350:h391. DOI:10.1136/bmj.h391 [ Links ]
35. Chao TE, Sharma K, Mandigo M, et al. Cost-effectiveness of surgery and its policy implications for global health: A systematic review and analysis. Lancet Glob Health 2014;2(6):e334-e345. DOI:10.1016/S2214-109X(14)70213-X [ Links ]
36. Costas-Chavarri A, Gillies R. Surgery for diabetes in low and middle-income countries. Lancet Diabetes Endocrinol 2014;2(7):534-535. DOI:10.1016/S2213-8587(14)70053-1 [ Links ]
37. Horsten G, Wise R, Ramroop S, Rodseth RN. The development of a scoring tool for the measurement of performance in managing hypotension and intra-operative cardiac arrest during spinal anaesthesia for caesarean section. S Afr J Anaesth Analg 2015;21(3):17-23. DOI:10.1080/22201181.2015.1054617 [ Links ]
38. Orkin FK. Risk stratification, risk adjustment, and other risks. Anesthesiology 2010;113(5):1001-1003. DOI:10.1097/ALN.0b013e3181f7ab17 [ Links ]
39. Steyerberg EW, Vergouwe Y. Towards better clinical prediction models: Seven steps for development and an ABCD for validation. Eur Heart J 2014;35(29):1925-1931. DOI:10.1093/eurheartj/ehu207 [ Links ]
 Correspondence:
Correspondence:
B M Biccard
bruce.biccard@uct.ac.za
Accepted 9 November 2015.
Appendix 1. Members of the SAPORG group
SAPORG Research Priorities Writing Committee: B M Biccard, C S Alphonsus, D G Bishop, L Cronje, H-L Kluyts, B Kusel, S Maswime, R Oodit, A R Reed, A M Torborg, R Wise, on behalf of the SAPORG national research priority-setting working group.
SAPORG national research priority-setting working group: T S Adams, G R Alexander, N L Allorto, C S Alphonsus, K Bester, B M Biccard, D G Bishop, C B Businge, J F Cardoso, S Chetty, E Coetzee, J F Coetzee, L Cronje, R A Duys, R A Dyer, Z Farina, M Flint, L M Fombad, P D Gopalan, R M Gray, T C Hardcastle, R Harrichandparsad, N D Hauser, M F M James, M A Jaworska, T Kisten, H-L Kluyts, M Z Koto, B S Kusel, G L Laing, C-A Lee, A I Levin, R L Llewellyn, T E Madiba, I A Maré, S Maswime, M G A Miller, D Mohr, S Z Molaoa, L F Montoya-Pelaez, Z Moolla, S T Mtshali, A L Myburgh, P H Navsaria, D C Nel, R Oodit, A R Reed, G Richards, D G D Richards, R N Rodseth, F Roodt, N F Rorke, C C Rout, D L Skinner, O Smith, S Spijkerman, S A Strachan, J L C Swanevelder, A Thotharam, A M Torborg, E W Turton, A van Niekerk, R P von Rahden, A Vorster, R D Wise.














