Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.106 n.4 Pretoria Apr. 2016
http://dx.doi.org/10.7196/samj.2016.v106i4.9950
RESEARCH
A deadly combination - HIV and diabetes mellitus: Where are we now?
S PillayI; C AldousII; F MahomedIII
IMB ChB, FCP (SA), MMed; Department of Internal Medicine, Edendale Hospital, Pietermaritzburg, South Africa
IIPhD; School of Clinical Medicine, University of KwaZulu-Natal, Durban, South Africa
IIIMB ChB, FCP (SA), FRCP, Cert Endocrinology; Department of Internal Medicine, Edendale Hospital, Pietermaritzburg, South Africa
ABSTRACT
BACKGROUND: The combination of HIV infection and diabetes mellitus (DM) represents a collision of two chronic conditions. Both HIV and DM increase the risk of developing tuberculosis (TB). Health resources in developing countries are already under strain as a result of the TB epidemic and poor diabetic control would further worsen this epidemic. Optimal diabetic control provides one avenue of curbing the TB epidemic in developing countries
OBJECTIVES: To establish if there is a difference in blood pressure, lipid and glycaemic control and complications between HIV-infected and uninfected diabetic patients; and to compare characteristics among HIV-infected diabetic patients between those with optimal and sub-optimal glycaemic control
METHODS: This was a retrospective chart review of all patients who visited the Edendale Hospital diabetic clinic, Pietermaritzburg, from 1 October 2012 to 30 September 2013
RESULTS: There were statistically significant differences noted in the following parameters between HIV-infected and uninfected diabetic patients: (i) mean HbA1c% (11.08% v. 10.14%, respectively); (ii) nephropathy defined by proteinuria (25.66% v. 15.43%); (iii) neuropathy (48.68% v. 42.10%); and (iv) Kidney Disease Outcomes Quality Initiative (KDOQI) stage >2 chronic kidney disease (30.87% v. 41.67%). There were no significant differences noted in the percentage of patients achieving the following target parameters between the two cohorts: (i) blood pressure (42.11% v. 35.62%); (ii) total cholesterol (36.84% v. 34.67%); and (iii) triglycerides (42.76% v. 40.19%). Within the HIV-infected diabetic cohort 85.23% displayed suboptimal glycaemic control. A significant percentage of HIV-infected diabetic patients on antiretroviral (ARV) therapy (89.36%) had suboptimal glycaemic control. HIV-infected female diabetic patients showed a significant increased waist circumference when compared with their HIV-uninfected counterparts
CONCLUSION: HIV-infected diabetic patients had significantly poorer blood sugar control and a higher incidence of neuropathy and nephropathy (when defined by overt proteinuria). There was a non-significant difference noted between the HIV-infected and uninfected diabetic patients with regard to blood pressure and lipid control. The majority of HIV-infected patients on ARVs failed to achieve target glycaemic control. Obesity remains a global challenge, as noted in both the HIV-infected and uninfected diabetic patients
HIV infection has reached epidemic levels, especially in sub-Saharan Africa. In South Africa (SA) the prevalence of HIV infection is approximately 10%.[1,2] Patients with HIV infection are living longer as a result of the availability and access to antiretroviral drugs (ARVs) and more chronic complications of HIV are therefore being seen. Diabetes mellitus (DM) is one such complication.[3] Both ARVs and the severity of HIV infection predispose patients to the development of DM.[3-5] HIV-infected patients are twice as likely to develop type 2 DM compared with HIV-uninfected individuals.[6] Both DM and HIV infection and/or ARV use can cause distal sensory peripheral neuropathy (DSP).[7,8] The combination of DM and HIV infection represents a collision of two chronic conditions.
SA has the third-highest incidence of tuberculosis (TB) worldwide. Approximately 1% of the population develops active TB annually.[9] Globally DM accounts for 15% of the aetiology of TB. DM increases the risk of developing TB three-fold.[6] Diabetic patients who contract TB generally have poorer outcomes in respect of higher relapse rates and increased risk of death from TB, compared with patients who have TB alone.[10] The TB epidemic is fuelled by the increased prevalence of HIV infection in SA.[11] The risk of developing TB increases from 5 - 10% in a lifetime in HIV-uninfected patients to 5 - 10% per year in HIV-infected patients.[12] The SA National Strategic Plan (NSP) aims to decrease the incidence and mortality of TB by half by 2016 and to completely eradicate new TB infections and deaths by 2032.[13] Both DM and HIV infection increase the risk of developing TB. Optimal control of DM and HIV is essential if we are to achieve the NSP goals and limit the spread of TB, thereby decreasing the enormous burden that TB places on the economies of developing countries. The objective of this study was two-fold:
1. To establish differences in the following parameters between the HIV-infected and uninfected diabetic patients:
- Glycaemic control
- Sitting blood pressure (BP)
- Mean total cholesterol and triglyceride levels
- Nephropathy
- Neuropathy
- Retinopathy
- Body mass index (BMI), waist circumference, glomerular filtration rate (GFR), vitamin B12 levels
- Pharmacological management.
2. To divide the HIV-infected diabetic cohort into those with and without optimal glycaemic control and then to compare the following characteristics between the 2 groups:
- Glycaemic control
- Duration of DM
- Sitting BP
- BMI
- Mean waist circumference
- Number of patients on ARVs
- Mean CD4 count
- Mean total cholesterol and triglyceride levels
- Mean GFR.
Our hypothesis was that HIV-infected diabetic patients had poorer control of the above clinical and biochemical variables and had a higher prevalence of diabetic complications when compared with their HIV-uninfected counterparts. The second hypothesis was that the majority of HIV-infected patients who had suboptimal control also had poor blood pressure and lipid control and were on ARVs.
Methods
This was a retrospective study assessing all patients seen at the Edendale Hospital diabetic clinic during the period 1 October 2012 to 30 September 2013. Edendale Hospital is a regional level hospital situated in Pietermaritzburg, the capital of KwaZulu-Natal. This clinic caters to both types 1 and 2 diabetic patients aged 13 years and older. In September 2012, a diabetic data sheet (Appendix 1) was introduced into the clinic. As part of the comprehensive assessment of these diabetic patients, a history of retroviral status, cluster of differentiation (CD4) count and ARV drugs were collected. The purpose of the data sheet was to ensure that there was standardisation in the approach to all diabetic patients seen at the clinic. All data were captured into a computerised program specifically designed for monitoring and research within the clinic. The program is designed to generate necessary statistics and graphs. Data of all patients seen during the study period were analysed and there were no exclusion criteria used for this study.
The Bio-Rad D-10 machine (Bio-Rad, USA) was used for analysing the HbA1c values at the laboratory. Both the laboratory and the machine are NGSP (National Glycohemoglobin Standardization Program) accredited to maintain standardisation of HbA1c results. The Adam BMI scale was used to measure height, weight and BMI readings (Model MDW-300L). GFR was calculated using the Modified Diet in Renal Disease (MDRD) formula: GFR=186 χ (creatinine (micromol/L)/88.4)-L154 χ (age)-0.203 χ (0.742 if female) χ (1.210 if black). According to the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines there are five stages of chronic kidney disease (CKD) based on calculation of GFR.[14]
Postural hypotension was identified when there was a drop in systolic BP by 20 mmHg or more and/or a drop in diastolic BP by 10 mmHg or more, and autonomic neuropathy was present if there was orthostatic hypotension and/or resting tachycardia (heart rate >100 beats per minute (bpm)). The fundoscopy findings were those documented by the Edendale Hospital ophthalmology clinic. Urine dipstick proteinuria using Makromed test strips was used as a surrogate marker for nephropathy. No microalbumin dipsticks were available for testing at the clinic.
The presence on history taking of numbness or pain, together with distal sensory loss and/or absent ankle reflexes on clinical examination, was used as a marker for DSP. [7]
South African Endocrine and Metabolic Society (SEMDSA) guidelines from 2012 were used to define the following goals:[15]
- HbA1c <7%
- Blood pressure <140/80 mmHg
- Total cholesterol level <4.5 mmol/L
- Triglyceride level <1.7 mmol/L.
Results
Epidemiology
There were 653 first-patient visits during the study period. More type 2 diabetic patients were seen (83.46%). One hundred and forty-nine patients (22.82%) self-reported being HIV-infected. Among the HIV-infected diabetic patients, 67.76% were female and the majority had type 2 diabetes (82.24%). One hundred and sixteen HIV-infected diabetic patients (77.85%) had had diabetes for a period of <10 years. The majority of HIV-uninfected diabetic patients (64.82% for type 1 DM and 68.07% for type 2 DM patients) had also had DM for <10 years. Just over half of the HIV-infected diabetic patients (54.36%) seen at the clinic were between 41 and 60 years of age. This differed from the HIV-uninfected diabetic patients who were older patients aged 51 - 70 years (50.36%).
Glycaemic control
The mean HbA1c achieved in the HIV-infected diabetic cohort v. the HIV-uninfected diabetic cohort was 11.08% v. 10.14% (p=0.044), respectively (Wilcoxon rank-sum test).
The risk of being HIV-infected for patients who had optimal HbA1c control was 1.34 times that of patients who didn't have optimal HBA1c control (odds ratio (OR) 1.34; 95% confidence interval (CI) 0.94 - 1.92). The odds of having HIV infection for patients with optimal HBA1c control was 1.50 times the odds for patients who did not have optimal HBA1c control (OR 1.50; 95% CI 0.89 - 2.53).
Blood pressure control
The mean BP obtained in the HIV-infected diabetic cohort was 146/90 mmHg (sitting) and 145/92 mmHg (standing). Target BP control in HIV-infected diabetic patients v. HIV-uninfected diabetic patients was achieved in 42.11% v. 35.62% (p=0.71), respectively (Fisher's exact test). The risk of being HIV-infected for patients who had optimal BP control was 1.06 times that of patients who didn't have optimal BP control (OR 1.06; 95% CI 0.81 - 1.39). The odds of having HIV infection for patients with optimal BP control was 1.08 times greater for patients who did not have optimal BP control (OR 1.08; 95% CI 0.75 - 1.56).
Lipid control
Target cholesterol level was achieved in 63.16% and target triglyceride levels were achieved in 57.24% of HIV-infected diabetic patients. The differences between HIV status and elevated cholesterol levels was not statistically significant (p=0.637, Fisher's exact test). The risk of being HIV positive for patients who had elevated cholesterol levels was 0.92 times that of patients who did not have elevated cholesterol levels (OR 0.92; 95% CI 0.69 - 1.22). The odds of having HIV infection for patients with elevated cholesterol levels was 0.90 times the odds for patients who did not have elevated cholesterol levels (OR 0.90; 95% CI 0.62 - 1.30). The differences between HIV status and elevated triglyceride levels was not statistically significant (p=0.582, Fisher's exact test). The risk of being HIV positive for patients who had elevated triglyceride levels was 0.92 times that of patients who didn't have elevated cholesterol levels (OR 0.92; 95% CI 0.70 - 1.21). The odds of having HIV infection for patients with elevated triglyceride levels was 0.90 times the odds for patients who did not have elevated cholesterol levels (OR 0.90; 95% CI 0.62 - 1.29). Table 1 summarises the comparison of those achieving lipid targets.

Diabetes complications
Table 2 illustrates that HIV-infected diabetic patients had significant higher prevalence of both nephropathy (when defined by overt proteinuria) and neuropathy when compared with their HIV-uninfected counterparts. However, the prevalence of CKD KDOQI stage 2 or greater was significantly higher in the HIV-uninfected cohort of diabetic patients (Tables 2 and 3).
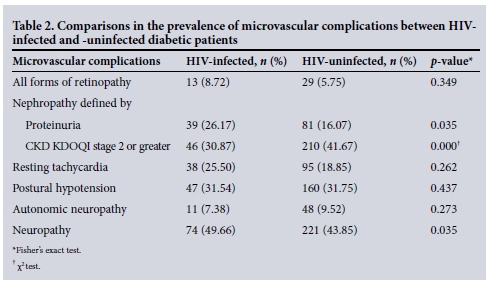
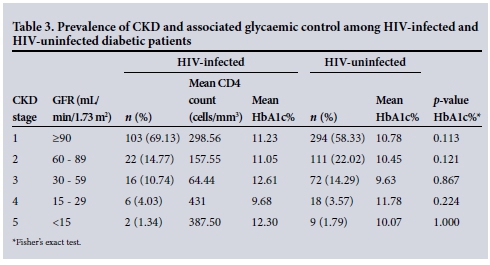
The mean GFR in our HIV-infected v. our HIV-uninfected diabetic patients was 124.89 mL/min/1.73 m2 v. 113.48 mL/min/1.73 m2 (p=0.048), respectively (Wilcoxon rank-sum test).
Waist circumference/BMI
More than half (55.91%) of the HIV-infected diabetic cohort had a BMI >30 kg/m2. The mean waist circumference was lower in HIV-infected v. HIV-uninfected diabetic males (97.41 cm v. 99.69 cm (p=1.61), respectively, Fisher's exact test)). There was a similar trend noted in HIV-infected v. HIV-uninfected diabetic females (106.65 cm v. 107.54 cm (p=0.55), respectively, Fisher's exact test). HIV-infected diabetic female patients with increased waist circumferences had poorer glycaemic control when compared with their counterparts with normal waist circumferences.
Vitamin B12 (cyanocobalamin) deficiency
Both HIV-infected and HIV-unifected diabetic patients had a high incidence of vitamin B12 deficiency (42.76% v. 44.57%, respectively). The difference between HIV status and vitamin B12 deficiency was not significant (p=0.145, Fisher's exact test).
ARVs and CD4 counts
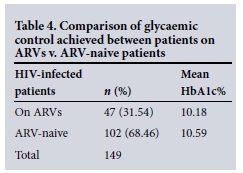
In this cohort of diabetic HIV-infected patients, 51.68% had a documented CD4 count, and the mean CD4 count was 491.45 (standard deviation (SD) 271.69) cells/mm3. Table 4 demonstrates that there was no significant difference noted between glycaemic control (HbA1c percentage) observed between patients on ARVs v. those that were ARV naive (10.18 v. 10.59, p=0.447, respectively) (Mann-Whitney test).
Pharmacological management and glycaemic control comparisons made between the HIV-infected and HIV-uninfected diabetic patients
HIV-uninfected patients on insulin monotherapy and those on combination of metformin and oral antidiabetic medicines (OADs) had significantly better glycaemic control than their HIV-infected counterparts. There was no statistical difference between the mean HbA1c percentage obtained in the HIV-infected cohort of diabetic patients who were on insulin monotherapy v. those who were on a combination of insulin and OADs (10.85% v. 11.76%, p=1.00, respectively) (Table 5).
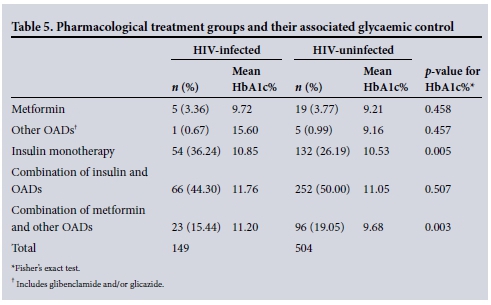
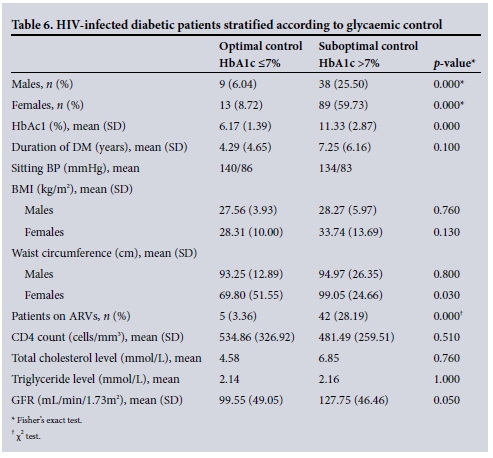
Table 6 shows observations noted when the HIV-infected diabetic patients were grouped into those with v. those without optimal glycaemic control.
Discussion
SA is burdened by the HIV and TB epidemics. In addition to this, the burden of communicable diseases such as DM, together with its long-term complications, weigh heavily on the economy of the country. Both HIV and DM increase the risk of developing TB, thereby worsening the burden that communicable diseases have on the country as well. Efforts need to be directed towards analysing the control that we are currently achieving in these HIV-infected diabetic patients. This study's objectives were to describe the control achieved in these HIV-infected diabetic patients when compared with their HIV-uninfected counterparts.
Glycaemic control achieved in the HIV-infected and HIV-uninfected diabetic patients
HIV-infected diabetic patients had significantly poorer control of their blood glucose
compared with their HIV-uninfected counterparts (mean HbA1c 11.08% v. 10.14%). Kim et al.[16] showed that the HbA1c values obtained in HIV-infected patients are lower than the actual value for a variety of reasons including higher mean corpuscular volume (MCV) and haemolysis. Therefore, our HbA1c of 11.08% is probably an underestimation of the actual value. This emphasises that our HIV-infected patients are not achieving optimal control. Only ~1 in 7 (14.76%) of our diabetic HIV-infected patients actually achieved target HbA1c levels. This is in stark contrast to other studies carried out abroad which show a 30 -67% target HbA1c attainment in their HIV-infected diabetic patients.[17,18]
The majority of the HIV-infected diabetic patients (85.23%) displayed suboptimal glycaemic control. A significant percentage of HIV-infected diabetic patients on ARVs (89.36%) failed to achieve optimal glycaemic control. HIV-infected female diabetic patients showed a significant difference in waist circumference when compared with their HIV-uninfected counterparts.
BP control
The United Kingdom Prospective Diabetes Study (UKPDS) established that intensive BP control in the management of DM forms an integral part of preventing complications.[8] Fewer than half (42.11%) of our HIV-infected
patients achieved target BP. This figure is much lower than that reported by Adeyemi et al.[17] (56%) in 2009, but similar to what Satlin et al.[18]found in their study published in 2011. The results from our study indicated no significant difference in BP control between the HIV-infected and HIV-uninfected patients.
Lipid control
A significant proportion of all diabetic patients (both HIV-infected and uninfected) seen at our clinic had hyperlipidaemia. Previous studies have shown an increased risk for metabolic syndrome in HIV-infected diabetic patients.[5] The majority of HIV-infected diabetic patients managed to achieve optimal lipid control. However, there was no significant difference in lipid control between the two cohorts.
Renal complications
HIV-infected diabetic patients showed a higher prevalence of nephropathy when nephropathy was defined using overt proteinuria diagnosed on urine dipstick measurement. Both HIV and DM increase the risk of developing proteinuria, chronic renal disease and renal failure.'191 Our cohort demonstrated this phenomenon, with 25.66% having overt proteinuria detected on routine urine dipstick testing. Renal disease in DM is an ever-present threat, more so for diabetic patients with concomitant HIV infection. Our study further demonstrated that the incidence of CKD based on GFR measurements was significantly higher in the HIV-unifected diabetic patient. The mean GFR was higher in HIV-infected diabetic patients. This is probably explained by the younger age range of the HIV-infected v. uninfected diabetic patients noted in our study (41 - 60 v. 51 - 70 years, respectively).
Neuropathy
The prevalence of neuropathy was significantly higher in the HIV-infected v. the un infected diabetic patients. As mentioned earlier DM, HIV infection and ARVs all increase the patient's risk of developing neuropathy.[7,8]
Management issues surrounding HIV-infected v. HIV-uninfected diabetic patients
An average waist circumference of >86 cm for males and >92 cm for females was used as an indicator of central obesity.[20] Although the HIV-infected cohort had a lower mean waist circumference than their HIV-uninfected counterparts, they still met the above criteria for central obesity in both males and females. This increased central obesity increases their risk of developing DM. Our HIV-infected cohort demonstrated a high incidence of obesity (55.91%); hence the need for reinforcement of lifestyle modification remains one of the cornerstones of modalities of treatment, irrespective of whether a patient is HIV-infected or not.
Metformin has been shown to improve insulin resistance in diabetic patients with HIV infection; however metformin use in HIV-infected patients has been associated with more cases of diarrhoea, decreases in subcutaneous fat and lactic acidosis.[3] Sulphonylureas are usually safe but their action may make them ineffective when there is severe insulin resistance. The best drug to treat DM in HIV-infected patients would be insulin as it has a positive growth effect and little or no drug interactions with ARVs and it can be used in patients with hepatic and renal dysfunction.[21,22] Just over a third of our patients (36.24%) were on insulin monotherapy and a further 44.30% were on a combination of insulin and OADs. Our results show that there was no significant difference in glycaemic control between those taking insulin monotherapy and those taking a combination of insulin and OADs within the HIV-infected cohort of patients. HIV-unifected patients on insulin monotherapy and those on metformin and other OADs had significantly better glycaemic control when compared with their HIV-infected counterparts.
Increased BMI, waist circumferences and low CD4 counts are associated with increased risk of development of DM in HIV-infected patients.'5,61 Our HIV-infected cohort had a relatively low mean (SD) CD4 of 228.94 (307.60) cells/mm3, which increased the possibility of developing DM.
Vitamin B12 deficiency
The study revealed that a significant percentage of our diabetic patients (both HIV-infected and HIV-uninfected) had vitamin B12 deficiency. However, there was no significant difference observed between the two groups of diabetic patients. Vitamin B12 deficiency in these patients warrants further investigation as the aetiology could be multifactorial, ranging from poor dietary intake and malabsorption syndromes associated with HIV infection to metformin-associated vitamin B12 deficiency.
Study limitations
HIV status of the patients required self-reporting and was probably underreported by patients owing to issues around social stigma.
Dates of diagnosis of HIV and initiation of ARVs were not routinely collected. This would have helped in establishing whether HIV and/or the ARVs prescribed could have potentiated the onset of DM in these patients.
The subgroup of nephropathy in HIV-infected diabetic patients involved only a small number of patients.
Conclusion
HIV-infected diabetic patients had significantly poorer glycaemic control with higher prevalences of complications (viz. neuropathy and nephropathy when defined by proteinuria) than their HIV-uninfected counterparts. Although no significant differences were noted between HIV-infected and HIV-unifected diabetic patients with regard to optimal BP achieved, a significant proportion (57.89%) of the HIV-infected patients failed to achieve optimal BP control. The majority of HIV-infected diabetic patients managed to achieve optimal lipid control. Within the group of HIV-infected patients who had suboptimal glycaemic control it was found that the majority were on ARVs and had suboptimal lipid control.
This study provides a baseline illustrating that we are achieving suboptimal glycaemic and BP control in our diabetic patients, both HIV-infected and uninfected. This spells disaster, as the combination of HIV and poor glycaemic control will increase the risk of these patients developing TB and will further worsen the current burden of communicable diseases on developing countries like SA. It is now our duty to work from this baseline to assist our patients in gaining better control of their diabetes and thus improving their quality of life.
References
1. UNAIDS. 2013. Spectrum Version 4.57. Geneva: United Nations, 2013. [ Links ]
2. Statistics South Africa. Mid-year population estimates 2013. May 2013. http://www.statssa.gov.za/publications/P0302/P03022013.pdf (accessed 16 January 2015). [ Links ]
3. Kalra S, Kalra B, Agrawal N, Unnikrishnan AG. Understanding diabetes in patients with HIV/AIDS. Diabetol Metab Synd 2011;3:2. [ Links ]
4. Kalra S, Sleim H, Kotwal N. Human immunodeficiency virus and the endocrine system. Indian J Endocrinol Metab 2011;15(4):231-233. DOI:10.4103/2230-8210.85566 [ Links ]
5. Kalra S, Agrawal N. Diabetes and HIV: Current understanding and future perspectives. Current Diabetes Rep 2013;13:419-427. DOI:10.1007/s11892-013-0369-9 [ Links ]
6. Galli L, Salpietro S, Pellicciotta G, et al. Risk of type 2 diabetes among HIV-infected and healthy subjects in Italy. Eur J Epidemiol 2012;27(8):657-665. DOI: 10.1007/s10654-012-9707-5 [ Links ]
7. Cherry CL, Wesselingh SL, Lal L, et al. Evaluation of a clinical screening tool for HIV- associated sensory neuropathies. Neurology 2005;65(11):1778-1781. DOI:10.1212/01.wnl.0000187119.33075.41 [ Links ]
8. American Diabetes Association. Implications of United Kingdom Prospective Diabetes Study. Diabetes Care 2002;Suppl 1:S28-S32. [ Links ]
9. South African Department of Health. Annual Performance Plan 2012/13 - 2014/15, Department of Health, South Africa 2012. www.tbfacts.org/App2012-2014.pdf (accessed 16 August 2015). [ Links ]
10. Lonnroth K, Roglic G, Harries AD. Improving tuberculosis prevention and care through addressing the global diabetes epidemic: From evidence to policy and practice. Lancet Diabetes Endocrinol 2014;2(9):730-739. DOI:10.1016/S2213-8587(14)70109-3 [ Links ]
11. World Health Organization. Global Tuberculosis Report 2012. http://www.who.int/tb/publications/global_report/gtbr12_main.pdf (accessed 16 August 2015). [ Links ]
12. World Health Organization. Implementing the WHO Stop TB Strategy: A Handbook for National Tuberculosis Control Programmes. Geneva, World Health Organization, 2008:67. www.who.int/tb/publications/2008/ (accessed 16 August 2015). [ Links ]
13. South African National Department of Health. National Strategic Plan on HIV, STIs and TB: 20122016. http://www.doh.gov.za/docs/stratdocs/2012/NSPfull.pdf (accessed 16 August 2015). [ Links ]
14. National Kidney Foundation. KDOQI Guidelines 2000. http://www.kidney.org/professionals/kdoqi/guidelines_ckd/p4_class g1.htm (accessed 17 January 2015). [ Links ]
15. Amod A, Ascott-Evans BH, Berg GI, et al. The 2012 SEMDSA guidelines for management of type 2 diabetes. J Endocrinol Metab Diabetes S Afr 2012;17(2):S4. [ Links ]
16. Kim PS, Woods C, Georgoff P, et al. A1C underestimates glycemia in HIV infection. Diabetes Care 2009;2(9):1591-1593. DOI:10.2337/dc09-0177. [ Links ]
17. Adeyemi O, Vibhakar S, Max B. Are we meeting the American Diabetes Association goals for HIV-infected patients with diabetes mellitus? Clin Infect Dis 2009;49:799-802. DOI:10.1086/605286 [ Links ]
18. Satlin MJ, Hoover DR, Glesby MJ. Glycemic control in HIV-infected patients with diabetes mellitus and rates of meeting American Diabetes Association management guidelines. AIDS Patient Care STDs 2011;25(1):5-12. DOI:10.1089/apc.2010.0237 [ Links ]
19. Kim PS, Woods C, Dutcher L, et al. Increased prevalence of albuminuria in HIV-infected adults with diabetes. PLoS One 2011;6(9);e24610. DOI:10.1371/journal.pone.0024610 [ Links ]
20. Motala AA, Esterhuizen T, Pirie FJ, Omar MAK. The prevalence of metabolic syndrome and determination of the optimal waist circumference cutoff points in a rural South African community. Diabetes Care 2011;34(4):1032-1037. DOI:10.2337/dc10-1921 [ Links ]
21. Reid MJA, Tsima BM, Kirk B. HIV and diabetes in Africa. Afr J Diabetes Med 2012;20(2):28-32. [ Links ]
22. Kalra S, Unnikrishnan AG, Raza SA, et al. South Asian consensus guidelines for the rational management of diabetes in human immunodeficiency virus/acquired immunodeficiency syndrome. Indian J Endocrinol Metab 2011;15(4):242-250. DOI:10.4103/2230-8210.85573 [ Links ]
 Correspondence:
Correspondence:
S Pillay
drspillay@iafrica.com
Accepted 5 October 2015.
Appendix 1.















