Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.106 no.2 Pretoria Fev. 2016
http://dx.doi.org/10.7196/SAMJ.2016.V106I2.9870
RESEARCH
Empirical antimicrobial therapy for probable v. directed therapy for possible ventilator-associated pneumonia in critically injured patients
Y RamsamyI, II, VI; D J J MuckartIII; J L BruceIV, V; T C HardcastleVII; K S S HanVIII, IX; K P MlisanaX, XI
IMB ChB, FCPath (Micro), MMed (Micro);Department of Medical Microbiology, Prince Mshiyeni Memorial Hospital, Durban, South Africa
IIMB ChB, FCPath (Micro), MMed (Micro); Department of Medical Microbiology, School of Laboratory Medicine and Medical Sciences, College of Health Sciences, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
IIIMB ChB, FRCS, MMSc Crit Care (SA); Department of Surgery, College of Health Sciences, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
IVMB ChB, FCS (SA)Trauma Fellow, College of Health Sciences, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
VMB ChB, FCS (SA) Pietermaritzburg Trauma Service, South Africa
VIMB ChB, FCPath (Micro), MMed (Micro); National Health Laboratory Service (KZN Academic Complex), Durban, South Africa
VIIMB ChB, MMed (Chir), FCS (SA), PhD; Department of Surgery, College of Health Sciences, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
VIIIMB BS, FCPath (Micro), MMed (Micro), DTMH, PDIC; Department of Medical Microbiology, School of Laboratory Medicine and Medical Sciences, College of Health Sciences, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
IXMB BS, FCPath (Micro), MMed (Micro), DTMH, PDIC; National Health Laboratory Service (KZN Academic Complex), Durban, South Africa
XMB ChB, MMed (Micro), PhD; Department of Medical Microbiology, School of Laboratory Medicine and Medical Sciences, College of Health Sciences, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
XIMB ChB, MMed (Micro), PhD; National Health Laboratory Service (KZN Academic Complex), Durban, South Africa
ABSTRACT
BACKGROUND: Ventilator-associated pneumonia (VAP) has recently been classified as possible or probable. Although direct attributable mortality has been difficult to prove, delay in instituting appropriate therapy has been reported to increase morbidity and mortality. Recent literature suggests that in possible VAP, instituting directed therapy while awaiting microbiological culture does not prejudice outcome compared with best-guess empirical therapy.
OBJECTIVES: To ascertain outcomes of directed v. empirical therapy in possible and probable VAP, respectively.
METHODS: Endotracheal aspirates were obtained from patients with suspected VAP. Those considered to have possible VAP were given directed therapy following culture results, whereas patients with more convincing evidence of VAP were classed as having probable VAP and commenced on empirical antimicrobials based on microbiological surveillance.
RESULTS: Pneumonia was suspected in 106 (36.8%) of 288 patients admitted during January - December 2014. Of these, 13 did not fulfil the criteria for VAP. Of the remaining 93 (32.2%), 31 (33.3%) were considered to have probable and 62 (66.7%) possible VAP. The former were commenced on empirical antimicrobials, with 28 (90.3%) receiving appropriate therapy. Of those with possible VAP, 34 (54.8%) were given directed therapy and in 28 (45.2%) no antimicrobials were prescribed. Of the latter, 24 recovered without antimicrobials and 4 died, 3 from severe traumatic brain injury and 1 due to overwhelming intra-abdominal sepsis. No death was directly attributable to failure to treat VAP. No significant difference in mortality was found between the 34 patients with possible VAP who were commenced on directed therapy and the 31 with probable VAP who were commenced on empirical antimicrobials (p=0.75).
CONCLUSIONS: Delaying antimicrobial therapy for VAP where clinical doubt exists does not adversely affect outcome. Furthermore, this policy limits the use of antimicrobials in patients with possible VAP following improvement in their clinical condition despite no therapy.
Ventilator-associated pneumonia (VAP) remains a diagnostic dilemma.[1] New-onset fever, leucocytosis, changes in chest auscultation, radiology and lung function and a positive bacterial culture suggest VAP, but do not necessarily confirm it. Overdiagnosis of VAP on clinical and radiological grounds may occur in up to 50% of patients, leading to inappropriate overuse of broad-spectrum antimicrobials, which is a major determinant of bacterial resistance, whereas failure to treat established VAP is reported to increase morbidity and mortality.[1] Guidelines continue to evolve as the definitions for VAP change in an effort to clarify a complex clinical condition and identify those patients who require therapy.[2] Recent Centers for Disease Control (CDC) guidelines[3] propose a tiered approach to VAP and the division of infection-related ventilator-associated complications into possible and probable VAP. A change in temperature and white blood cell count, worsening oxygenation, purulent secretions and the empirical prescription of antimicrobials are prerequisites for both categories. In addition, a diagnosis of possible VAP requires either purulent secretions or a positive bacterial culture of sputum, whereas a diagnosis of probable VAP requires purulent secretions and specific quantitative analysis of either an endotracheal aspirate (ETA) or more invasive specimens from bronchoalveolar lavage, a protected specimen brush, pleural fluid or lung tissue. In the absence of purulent secretions, diagnostic tests for Legionella or a number of viruses are recommended. In both possible and probable VAP, specimens containing normal respiratory or oral flora, Candida, coagulase-negative staphylococci or enterococci are excluded. Given the absolute criterion that antimicrobials need to be administered in both categories, these divisions do not reduce unnecessary prescriptions and will have little impact on curbing bacterial resistance.
Whether possible or probable, VAP is defined as pneumonia occurring after 48 hours of endotracheal intubation and mechanical ventilation. The American Thoracic Society guidelines[4] divide VAP into early (starting on days 3 and 4) and late (starting on day 5 or thereafter). Regardless of the time of onset of suspected VAP, initial antimicrobial therapy will of necessity be empirical until microbiology results become available. The recommendation is to use broad-spectrum drugs and de-escalate when sensitivities are reported.[5] Delays in microbiology results or marked improvement in the patient's clinical condition may perpetuate use of the initial choice of antimicrobial, a potent stimulus for multidrug bacterial resistance. Recent work suggests that where there is diagnostic doubt, delaying antimicrobials until culture results are available does not increase mortality and in fact may improve survival.[6]
The antimicrobial policy in the Trauma Intensive Care Unit (TICU) at Inkosi Albert Luthuli Central Hospital (IALCH), Durban, South Africa, is to treat probable VAP empirically but to await microbiological results before treating possible VAP. We embarked on a prospective observational study in the TICU with a view to determining whether delaying antimicrobial prescriptions in patients with possible VAP until microbiology results became available made any difference to outcome.
Methods
The study was approved by the Bioethics Committee of the University of KwaZulu-Natal, Durban (BE 207/09), as part of the class approval covering the unit data. All patients admitted to the TICU at IALCH who required mechanical ventilation for >48 hours were considered for inclusion in the study, which was conducted prospectively for the 12-month period January -December 2014. Patients in whom aspiration was suspected before or during endotracheal intubation, those in whom a positive culture was obtained within the first 48 hours, and those who did not conform to the CDC criteria were excluded. The diagnostic criteria for probable VAP were the combination of new-onset fever of >38.4oC, purulent secretions in an ETA, changes in chest auscultation or radiographs, a rise in the white cell count, a deterioration in lung function manifested by a reduction in the partial pressure of arterial oxygen/fraction of inspired oxygen ratio, reduced compliance and an elevated procalcitonin level. Possible VAP was defined similarly, but without marked changes in chest auscultation, radiology or pulmonary function. Early VAP was defined as occurring on the 3rd or 4th days of mechanical ventilation and late VAP as starting on or beyond day 5. All microbiology specimens were submitted as ETAs, and more invasive diagnostic tests were not undertaken. Specimens were processed by the National Health Laboratory Service (NHLS), where processing, identification of pathogens and susceptibility testing were conducted according to standard NHLS operating procedures.[7] Patients who fulfilled the criteria for probable VAP were commenced on empirical antimicrobial therapy as per the antimicrobial protocol in the TICU at IALCH, which is based on microbiological surveillance. The antimicrobial of choice for early-onset VAP is amoxicillin/clavulanic acid and that for late-onset VAP piperacillin/ tazobactam. Combination therapy is not used. Antimicrobial therapy for patients with possible VAP was delayed until microbiology results were available. Patients with Acinetobacter isolates were not treated unless this was the sole pathogen in a case of possible VAP, when nebulised amikacin was the treatment of choice. Data were analysed according to the first episode of suspected VAP with the hypothesis that delays in initiating antimicrobials at this time would affect outcome.
Endpoints of the study were duration of mechanical ventilation, length of stay in the TICU and mortality rate. Categorical data were analysed using the Χ2 or Fisher's exact tests and continuous data using Student's t-test. Differences in outcome between patients with possible and probable VAP were considered significant at p<0.05.
Results
A total of 288 patients were admitted to the TICU at IALCH during the study period, of whom 106 (36.8%) were suspected to be developing pneumonia. There were 84 (79.2%) males and 22 (20.8%) females, with a median age of 29 years (interquartile range (IQR) 21 - 37) and a median injury severity score (ISS) of 31 (IQR 24 - 38). The mechanism of injury was predominantly blunt, with 77 (72.6%) having been injured in motor vehicle collisions, 17 (16.1%) having sustained non-vehicular blunt trauma, and 8 (7.5%) having gunshot wounds, 3 (2.8%) stab wounds and 1 (0.9%) a snakebite. A total of 247 positive cultures were obtained, of which 168 (68.0%) were Gram-negative, 58 (23.4%) Gram-positive and 21(8.5%) fungal. Eleven different species of Gram-negative and 7 of Gram-positive organisms were cultured. Of the 106 patients, 13 did not fulfil the criteria for VAP (in 8 VAP was suspected <48 hours after intubation and mechanical ventilation, in 3 respiratory flora were isolated after suspected aspiration, and in 2 Candida was the sole isolate). These were excluded from analysis, leaving a total of 93 patients. Of these 93, 57 (61.3%) had a single episode of suspected VAP, 22 (23.6%) two episodes and the remaining 14 (15.1%) three or more episodes, with a maximum of five in a patient ventilated for 72 days. Based on the first episode of VAP, 62 patients (66.7%) were diagnosed as having possible and 31 (33.3%) probable VAP. The distribution and outcomes of possible and probable and early and late VAP are shown in Table 1.
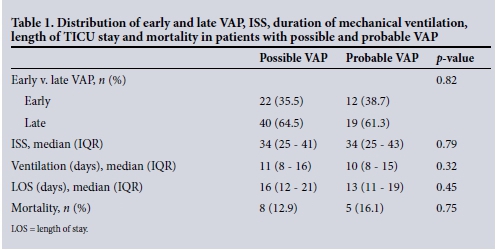
The commonest Gram-positive and Gram-negative pathogens in early and late VAP are documented in Table 2, and their susceptibilities in Tables 3 and 4.
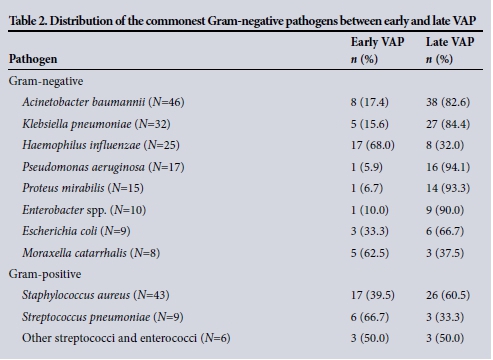
The most prevalent Gram-negative organism in early VAP was Haemophilus influenzae (38%). All isolates were susceptible to amoxicillin/clavulanate. In addition to being the most common isolate in late VAP, A. baumannii was the most prevalent Gram-negative isolate overall and almost five times more common in late as opposed to early VAP. Forty-three (93.4%) of the 46 isolates of A. baumannii were multidrug resistant, susceptible only to colistin (100%) and amikacin (96%). Of the 43 patients in whom multidrug-resistant (MDR) A. baumannii was cultured, 6 (14%) were offered therapy, 5 with nebulised amikacin and 1 with intravenous colistin. All but 1 patient with MDR A. baumannii survived, the sole death occurring in a patient with overwhelming uncontrolled abdominal sepsis, retroperitoneal necrotising fasciitis and multiple organ failure.
Of the 32 K. pneumoniae isolates, 28 (87.5%) were susceptible to amoxicillin/ clavulanate, 3 (9.3%) were extended-spectrum beta-lactamase (ESBL)-positive and susceptible to ciprofloxacin, and one was susceptible only to meropenem. There were 9 E. coli isolates, of which 2 were ESBL-positive and 7 were susceptible to amoxicillin/clavulanate. Of the 15 isolates of P. mirabilis, 4 (26.7%) were ESBL-positive and 11 (73.3%) were susceptible to amoxicillin/clavulanate. There were no MDR P. aeruginosa isolates; all were susceptible to piperacillin/tazobactam, ciprofloxacin and meropenem. All 10 Enterobacter spp. were susceptible to ciprofloxacin and meropenem. Serratia spp., Aeromonas spp. and Citrobacter spp. were rare isolates.
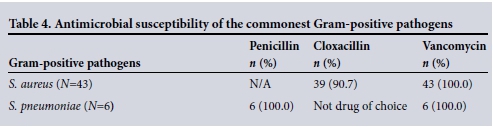
Among the Gram-positive organisms there were 43 isolates of S. aureus, of which 4 were vancomycin-sensitive methicillin-resistant S. aureus (MRSA) and arose during episodes of late VAP. All 6 isolates of S. pneumoniae were susceptible to penicillin.
Thirty-one patients were treated empirically for probable VAP, prescriptions being correct in 28 cases (90.3%). Among the 3 patients with incorrect prescriptions, ESBL K. pneumoniae resistant to amoxicillin/ clavul anate was isolated in 1 patient with early VAP, and E. aerogenes resistant to piperacillin/tazobactam was cultured in 2 patients with late VAP. In this group, 1 death occurred in an elderly man with severe traumatic brain injury and major blunt thoracic and abdominal trauma. Although inappropriate therapy may have contributed to his death, age and the extent of injury placed him in the probable non-survivor category. In the probable VAP cohort, no deaths were attributable to withdrawal of non-beneficial therapy due to futility.
Antimicrobials were commenced in 34 (54.8%) of the 62 patients with possible VAP, and not prescribed in the remaining 28 (45.2%). Of the latter, 24 recovered without antimicrobials and 4 died, no deaths being directly attributable to failure to treat nosocomial pneumonia. Death was due to withdrawal of non-beneficial therapy in 3 patients with severe traumatic brain injury and to retroperitoneal fasciitis in the remaining patient following the breakdown of a colonic anastomosis.
There was no significant difference in mortality between the 34 patients with possible VAP who were commenced on directed therapy and the 31 with probable VAP who were commenced on empirical antimicrobials (11.7% v. 16.1%; p=0.79). Excluding the 4 deaths due to withdrawal of non-beneficial therapy and uncontrolled abdominal sepsis, the overall mortality rate in patients with possible VAP, including those who were not offered antimicrobials, was 6.4% v. 16.1% in the probable group (p=0.29).
Discussion
Antimicrobials are an integral component of the management of sepsis,[5] and virtually all agree that the sooner appropriate antimicrobials are given, the better the outcome is. In addition to a delay in antimicrobial therapy, inappropriate prescriptions to which the pathogen is resistant are associated with decreased survival.[8] The recommendation is therefore to commence early broad-spectrum empirical therapy while awaiting microbiological confirmation, and then de-escalate as necessary.[9] Although minimising inappropriate prescriptions, this policy risks creating collateral damage and inducing bacterial resistance. Based on data showing that early appropriate administration of antimicrobials improves outcome in severe sepsis and septic shock, the same premise has been extrapolated to less-severe infections without proof of benefit. In patients with uncomplicated sepsis, the evidence for the timing of antimicrobial administration is contentious[10] and there are no studies substantiating the need for the same approach as is used in severe sepsis or septic shock. In fact, evidence to the contrary has been presented.[11] In the absence of haemodynamic instability or worsening organ function, there is no reported relationship between the timing of treatment and outcome. Moreover, the unwarranted use of broad-spectrum empirical antimicrobials in sepsis without organ dysfunction or hypotension may potentiate bacterial resistance without conferring benefit. Awaiting microbiological confirmation of the inciting pathogen and using directed therapy appears to be acceptable practice.
The pathogens isolated in our study were similar to those previously reported for VAP in the critically injured,[12] where Gram-negative organisms of the Enterobacteriaceae family predominated, especially in late VAP. Gram-positive species were less common, S. aureus being isolated most frequently. Among the pathogens we encountered few ESBL-producing organisms or MRSA, for which there are a number of explanations. Firstly, we have a strict infection control policy. Secondly, we have adopted a policy of antimicrobial stewardship and prescriptions based on microbiological surveillance. Thirdly, our patient population is young and antimicrobial naive; at the time of the first episode of VAP, only 40% had received prior antimicrobials.
Patients treated in the TICU are a unique population compared with those admitted to general medical and surgical intensive care units (ICUs). The TICU is exclusively for critically injured patients who require mechanical ventilation and are generally an otherwise well population free from chronic medical conditions and with no previous hospital visits or use of antimicrobials. Owing to ethical constraints regarding informed consent, and the knowledge that it does not impact on the immediate management or outcome of life-threatening injuries,[13] routine HIV testing is not undertaken.[14]
Although the isolated flora may be similar in ICUs, reasons for bacterial resistance to antimicrobials vary, the commonest being previous antimicrobial exposure, the overuse of broad-spectrum agents and selective pressure. Despite suggestions that the differentiation between early and late VAP is irrelevant and that all patients should be treated with broad-spectrum agents,[15] our data confirm that this distinction remains useful when deciding on empirical therapy. The vast majority of pathogens in early VAP in our patients were community-acquired flora susceptible to amoxicillin/ clavulanate, and indiscriminate use of broad-spectrum antimicrobials would encourage development of resistant strains.
MDR A. baumannii was the most common organism isolated in late VAP. Owing to its natural multidrug resistance and low virulence and pathogenicity, the unit policy is not to treat MDR Acinetobacter unless it is the sole pathogen in possible VAP that fails to improve. Of the 43 patients in whom MDR A. baumannii was cultured, only 6 were offered therapy, mainly in the form of inhaled/nebulised amikacin. There was 1 fatality due to uncontrolled retroperitoneal fasciitis following a faecal leak. Based on these data, we suggest that treating all Acinetobacter isolates is unnecessary and will promote bacterial resistance.
The high incidence of possible pneumonia in our cohort reflects the difficulties in confirming the diagnosis of VAP. This is especially problematic in patients with blunt thoracic trauma and the systemic inflammatory response syndrome. Fever, progressive changes in chest auscultation and radiographs and a positive sputum culture are common before resolution of the underlying lung injury. That said, if there is diagnostic doubt and no immediate need for therapy, our results confirm that in patients with possible VAP, awaiting definitive microbiologal results before commencing antimicrobials does not compromise outcome. Furthermore, due to an improvement in clinical signs in almost 50% of patients with possible VAP, antimicrobials were not prescribed. However, this policy may only be implemented in association with an effective microbiology service employing urgent Gram staining and rapid susceptibility testing. In addition, although the incidence of VAP is reported to be significantly higher in the critically injured, the outcome is better than for non-trauma patients.[16] The most likely explanation is that young, previously healthy males comprise the vast majority of the trauma population, whereas non-trauma patients are an older population, often with comorbidities. In that light, our findings may not be universally applicable to all critically ill populations.
A number of myths concerning antimicrobials perpetuate the prescription of broad-spectrum drugs. It is a misconception that sicker patients require what are erroneously termed 'stronger antibiotics'. Firstly, regardless of the severity of the underlying disease, the susceptibility of pathogens remains identical for that pathology. For example, the organisms involved in appendicitis are the same whether the disease is localised or causing generalised peritonitis, and as such there is no need initially to use a broader-spectrum agent. The same premise holds true for early VAP, where H. influenzae is almost universally susceptible to amoxicillin/clavulanate. Secondly, with the exception of bacteriostatic v. bactericidal antibiotics, there is no such entity as a stronger antibiotic, only antibiotics with a broader spectrum of action. To minimise the development of antimicrobial resistance, the principle should be to use a drug with the narrowest spectrum against the most likely pathogens. This requires a working knowledge of local flora and of their antibiotic sensitivities, achieved through bacteriological surveillance. Thirdly, there is no proof that empirical combination therapy has any advantage over monotherapy if an appropriate single antimicrobial is selected.[17] The only patients in whom combination therapy appears to be beneficial are those in septic shock.[9] In the presence of effective microbiological surveillance, it is possible to achieve adequate empirical antimicrobial therapy over 90% of the time with the initial empirical choice of a single agent.[18] The TICU at IALCH subscribes to stewardship and employs an empirical antimicrobial policy based on surveillance. Lastly, there is a mistaken belief that newer drugs are superior to their elderly counterparts. This is untrue - the drug of choice for a penicillin-sensitive organism is penicillin.
Combating the problem of drug resistance requires a 'multipronged' approach. In addition to antibiotic stewardship and surveillance, early convincing diagnosis of infection would greatly improve use of antibiotics and in turn address the problem of antibiotic resistance. Conventional microbiological culture, performed by most laboratories worldwide, requires several days for isolation, identification and antimicrobial susceptibility testing of the pathogen. As a result there is a delay in the identification of resistant bacteria, which results in the inappropriate use of antimicrobials. Rapid diagnostic methods must therefore have the capability of influencing early antibiotic decision-making to allow for more appropriate treatment of antibiotic-resistant bacterial infection, as well as minimisation of unnecessary use of broad-spectrum agents.[19] May et al.[20] described a novel strategy for the rapid diagnosis of VAP, using exhaled breath condensate fluid obtained from heat-moisture exchangers to provide a substrate for testing with the polymerase chain reaction to identify bacterial DNA. The advantage of molecular diagnostic platforms lies in the ability to diagnose pathogens and their accompanying resistance genes rapidly. The challenge to ICU clinicians is how to use antimicrobials most effectively to maximise patient benefits while minimising the emergence of resistance. In this, the use of rapid diagnostics may hold the key.[19]
Despite numerous warnings in the medical literature about the threat of bacterial resistance, indiscriminate antimicrobial prescribing continues unabated, with as many as 64% of prescriptions being deemed either unnecessary or an inappropriate choice.[21] Our data confirm that delaying antimicrobial prescriptions in situations of doubtful sepsis, or omitting therapy altogether, does not adversely affect outcome. The real possibility of a post-antibiotic era in the 21st century has been highlighted by the World Health Organization (WHO) report on antimicrobial resistance,[22] and concern has now reached political level[23,24] with proposals to provide incentives to the pharmaceutical industry to develop new antimicrobials. This approach is short sighted and ineffective for a number of reasons. History will undoubtedly repeat itself: the development of each new antimicrobial has been followed by resistance, the normal evolutionary process of mutation and natural selection conferring a survival benefit. In the modern pharmaceutical market, antimicrobials are not a cost-effective investment for research and development and, ironically, pressure from the pharmaceutical industry to use their broad-spectrum products has contributed to the current situation. The most logical and practical short- and long-term solutions to preserve what drugs we currently possess are education, bacteriological surveillance and antimicrobial stewardship.[18] Antimicrobial stewardship is of paramount importance, especially in areas with frequent antimicrobial use. Pivotal to success are interested clinicians and microbiologists, knowledge of local resistance patterns, and an antimicrobial policy that optimises the choice, dose and duration of therapy.[18] The particular patient population, local epidemiology, and prior antimicrobial exposure in each specific ICU need to be considered when considering empirical antimicrobial therapy.
The WHO has warned that bacterial resistance has become a global health emergency, and in the not-too-distant future there is the very real possibility that previously treatable common infections and minor injuries may become fatal. Unless urgent action is taken, a bacterial apocalyptic fantasy may become reality.[22]
References
1. Kollef MH. Ventilator-associated complications, including infection-related complications. Crit Care Clin 2013;29(1):33-50. [http://dx.doi.org/10.1016/j.ccc.2012.10.004] [ Links ]
2. Munro N, Ruggiero M. Ventilator-associated pneumonia bundle reconstruction for best care. AACN Advanced Critical Care 2014;25(2):163-175. [http://dx.doi.org/10.1097/NCI.0000000000000019] [ Links ]
3. Magill SS, Klompas M, Balk R, et al. Developing a new national approach to surveillance for ventilator-associated events. Am J Crit Care 2013;22(6):469-473. [http://dx.doi.org/10.4037/ajcc2013893] [ Links ]
4. American Thoracic Society. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Care Med 2005;171(4):388-416. [http://dx.doi.org/10.1164/rccm.200405-644ST] [ Links ]
5. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock. Crit Care Med 2013;41(2):580-563. [http://dx.doi.org/10.1097/CCM.0b013e31827e83af] [ Links ]
6. Hranjec T, Sawyer RG. Conservative initiation of antimicrobial treatment in ICU patients with suspected ICU-acquired infections: More haste less speed. Curr Opin Crit Care 2013;19(5):461-464. [http://dx.doi.org/10.1097/MCC.0b013e328364d525] [ Links ]
7. National Health Laboratory Service. Q-pulse Standard Operating Procedures Q-pulse 5/docs/active/ MIC 1569v1, MIC 1587v1, MIC 1840v1, MIC 1849v1. Johannesburg: NHLS, 2010. [ Links ]
8. Swanson JM, Wells DL. Empirical antibiotic therapy for ventilator-associated pneumonia. Antibiotics 2013;2(3):339-351. [http://dx.doi.org/10.3390/antibiotics2030339] [ Links ]
9. Borgatta B, Rello J. How to approach and treat VAP in ICU patients. BMC Infect Dis 2014;14:211. [http://dx.doi.org/10.1186/1471-2334-14-211] [ Links ]
10. Simonetti A, Viasus D, Garcia-Vidal C, et al. Timing of antibiotic administration and outcome of hospitalized patients with community-acquired and healthcare-associated pneumonia. Clin Microbiol Infect 2012;18(11):1149-1155. [http://dx.doi.org/10.1111/j.1469-0691.2011.03709.x] [ Links ]
11. De Groot B, Ansems A, Gerling DH, et al. The association between time to antibiotics and relevant clinical outcomes in emergency department patients with various stages of sepsis: A prospective multi-center study. Crit Care 2015;19:194 [http://dx.doi.org/10.1186/s13054-015-0936-3] [ Links ]
12. McMillian WD, Bednarik JL, Aloi JJ, et al. Utility of ampicillin-sulbactam for empiric treatment of ventilator-associated pneumonia in a trauma population. J Trauma 2010;69(4):861-865. [http://dx.doi.org/10.1097/TA.0b013e3181e83f8b] [ Links ]
13. Bhagwanjee S, Muckart DJJ, Jeena PM, et al. Does HIV status influence the outcome of patients admitted to a surgical intensive care unit? BMJ 1997;314:1077-1081. [http://dx.doi.org/10.1136/bmj.314.7087.1077a] [ Links ]
14. Kale R. Commentary: Failing to seek patients' consent to research is always wrong. BMJ 1997;314:1081. [http://dx.doi.org/10.1136/bmj.314.7087.1081] [ Links ]
15. Gastmeier P, Sohr D, Geffers C, et al. Early- and late-onset pneumonia: Is this still a useful classification? Antimicrob Agents Chemother 2009;53(7):2714-2718. [http://dx.doi.org/10.1128/AAC.01070-80] [ Links ]
16. Cook A, Norwood S, Berne J. Ventilator associated pneumonia is more common and of less consequence in trauma patients compared with other critically ill patients. J Trauma 2010;69(5):1083-1091. [http://dx.doi.org/10.1097/TA.0b013e3181f9fb51] [ Links ]
17. Kumar A. An alternate physiological paradigm of sepsis and septic shock. Virulence 2014;5(1):80-97. [http://dx.doi.org/10.4161/viru.26913] [ Links ]
18. Ramsamy Y, Muckart DJJ, Han KSS. Microbiological surveillance and antimicrobial stewardship minimise the need for ultrabroad-spectrum combination therapy for treatment of nosocomial infections in a trauma intensive care unit: An audit of an evidence-based empiric antimicrobial policy. S Afr Med J 2013;103(6):371-376. [http://dx.doi.org/10.7196/SAMJ.6459] [ Links ]
19. Kollef MH. Ventilator-associated pneumonia: The role of emerging therapies and diagnostics. Chest 2015;147(6):1448-1450. [http://dx.doi.org/10.1378/chest.14-2745] [ Links ]
20. May AK, Brady JS, Romano-Keeler J, et al. A pilot study of noninvasive assessment of the lung microbiota as a potential tool for the early diagnosis of ventilator-associated pneumonia. Chest 2015;147(6):1494-1502. [http://dx.doi.org/10.1378/chest.14-1687] [ Links ]
21. Cusini A, Rampini SK, Bansal V, et al. Different patterns of inappropriate antimicrobial use in surgical and medical units at a tertiary care hospital in Switzerland: A prevalence survey. PLoS One 2010;5(11):e14011. [http://dx.doi.org/10.1371/journal.pone.0014011] [ Links ]
22. World Health Organization. Antibiotic Resistance Global Report on Surveillance. Geneva: WHO, 2014. [ Links ]
23. Report to the President on Combating Antibiotic Resistance. President's Council of Advisors on Science and Technology (PCAST). 2014. www.whitehouse.gov/ostp/pcast (accessed 18 September 2014). [ Links ]
24. BBC News Health. Antibiotic resistance: Cameron warns of medical 'Dark Ages. http://www.bbc.com/news/health/-28098838 (accessed 2 July 2014). [ Links ]
 Correspondence:
Correspondence:
Y Ramsamy
yogandree@gmail.com
Accepted 29 October 2015.














