Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.106 n.2 Pretoria Feb. 2016
http://dx.doi.org/10.7196/SAMJ.2016.V106I2.9928
IN PRACTICE
An effective approach to chronic kidney disease in South Africa
M R MoosaI; A M MeyersII; E GottlichIII; S NaickerIV
IMB ChB, FCP (SA), MD, FRCP (Lond); Ministerial Advisory Committee on Transplantation, Executive Head of Medicine, Faculty of Medicine and Health Sciences, Stellenbosch University, Tygerberg, Cape Town, South Africa, and a specialist nephrologist at Tygerberg Academic Hospital, Cape Town
IIMB BCh, FCP (SA), FRCP (Lond); Professor of Medicine in the Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa, and Chairman of the National Kidney Foundation
IIIMB B Ch, DCH, FCP Paeds (SA), Cert Nephrol (Paeds) (SA); Paediatric nephrologist at Morningside Mediclinic, Johannesburg
IVMB ChB, MRCP (UK), FRCP (Lond), PhD; Ministerial Advisory Committee on Transplantation and Emeritus Professor of Medicine in the Faculty of Health Sciences, University of the Witwatersrand
ABSTRACT
Very few patients with end-stage kidney disease in South Africa receive renal replacement treatment (RRT), despite the rapidly growing demand, because of resource constraints. Nephrologists who agonise daily about who to treat and who not to, and have been doing so since the inception of dialysis in this country, welcomed the opportunity to interact with the National Department of Health at a recent summit of stakeholders. The major challenges were identified and recommendations for short- to long-term solutions were made. While the renal community can still improve efficiencies, it is clear that much of the responsibility for improving access to RRT and reducing inequities must be borne by the national government. The summit marks the first step in a process that we hope will ultimately culminate in universal access to RRT for all South Africans.
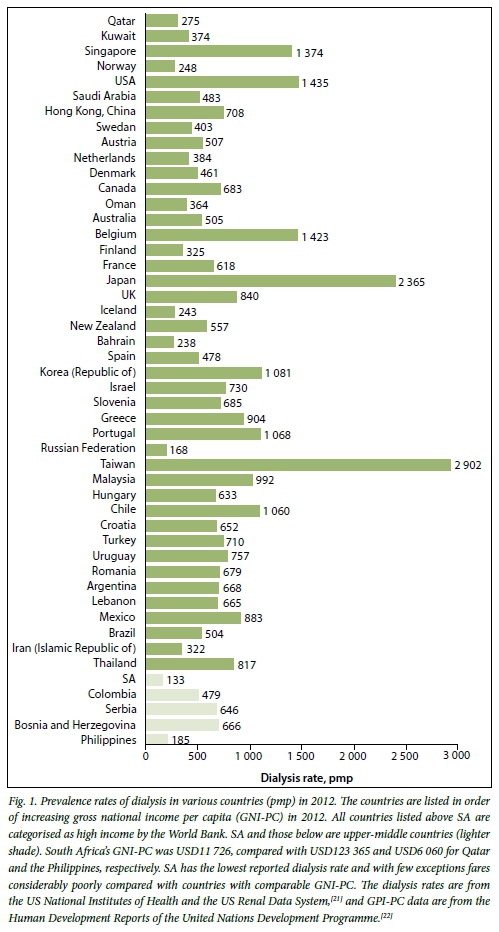
Fewer than 5% of all patients with end-stage kidney disease (ESKD) in sub-Saharan Africa receive dialysis, with patients in several countries having no access at all.[1] While the situation is somewhat less dire in South Africa (SA), we compare very poorly with countries that are our economic peers (Fig. 1). The recent release of the South African Renal Registry by the South African Renal Society[2] produced data that were so alarming that the National Department of Health (NDoH) convened a national summit to discuss the challenges faced in SA. Delegates to the summit included relevant stakeholders: public and private sector clinicians, healthcare funders, representatives of the pharmaceutical industry and the NDoH, a representative of the World Health Organization (WHO) and representatives of the National Kidney Foundation. The meeting was held in Johannesburg over 2 days in March 2015 and produced recommendations to provide short-, medium- and long-term solutions. Discussions revolved around a few key issues in an attempt to find workable solutions.
Human resources
Chronic kidney disease (CKD) affects 14% of the adult population in sub-Saharan Africa.[1] The vast majority of South Africans who have ESKD die because of lack of access to definitive lifesaving treatment.[3] The major challenge faced by the country is lack of sufficient resources - capital and human - to provide universal access. The reason why SA has lagged so severely behind in the provision of renal replacement treatment (RRT) compared with similar middle-income countries is probably the HIV/AIDS epidemic, which has demanded a disproportionate quantum of the health budget.[4] In addition, there are inequities in the provision of renal services at several levels. Poorer patients and patients in rural areas are under-served as a result of the lack of facilities. In response to RRT being made a minimum prescribed benefit, the private sector facilities have grown by over 3 000% over two decades, but in contrast there has been no significant growth in renal services in the public sector that serves over 80% of the country's population.[2] The renal community faces a major shortage of skilled personnel and reflects the national skills challenges.[5]
The lack of appropriate and adequate skilled personnel has hampered the development of renal care in SA. Insufficient numbers of personnel are being trained and effective retention strategies are lacking. To address the situation, the summit proposed medium- and long-term strategies. In order to ensure high-quality renal care it was agreed that a nephrologist (or a specialist physician where no nephrologist is available) should be attached to every dialysis unit. SA currently has 1.1 nephrologists per million population (pmp), compared with 6.5 and 4.5 in Egypt and Morocco, respectively - not even comparing ourselves with high-income countries.[6] Additional posts to train more specialist nephrologists were strongly recommended. This would also require an increase in the number of permanent academic hospital posts and a clear retention strategy. A mid-level worker, provisionally identified as a 'clinical associate' working under the supervision of a nephrologist, was proposed as an option. The clinical associate would be trained to perform procedures that would obviate the need to transfer patients to the care of a nephrologist, including insertion of dialysis catheters and performing renal biopsies. The role of this associate will need further discussion to ensure that the level of training is matched to the requisite skills level.
Renal nurses form the backbone of any renal replacement programme, but are in short supply. An important approach is to create more training centres and programmes for renal nurses around the country. A way of optimising the use of the limited numbers of renal nurses is to reduce the recommended dialysis staff-to-patient ratio from the current 1:4 to 1:6, as a strategy that would be easily and rapidly implemented. While this may raise some concerns, these ratios do prevail in dialysis units in high-income countries.[7] The risks are those of greater burnout and reducing the quality of care, although there are limited data to support this as yet.[8] However, with the current generation of dialysis machines equipped with vastly improved technology and safety features, a different level of nursing oversight is required. Reducing the staffing ratio may allow additional dialysis sessions to be activated without additional infrastructure. However, the summit made the crucial suggestion that a completely different staffing model be considered: dialysis units should be staffed with registered nurses who take responsibility for up to 16 patients with the development of a team of mid-level workers specifically trained to provide dialysis-related services who would work under the supervision of the registered nurse.[9] In this way, staff-to-patient ratios of 1:4 could be retained but costs contained, as fewer registered nurses would be required. The summit recommended a staff-to-patient ratio for peritoneal dialysis of 1:25.
The summit also recognised that a successful renal programme required the services of other skilled personnel, including surgeons (trained in fashioning vascular access and placement of peritoneal dialysis catheters) and dialysis technicians (whose scope of practice must be broadened to assist the registered nurses), alongside social workers, dieticians and transplant co-ordinators, among other support staff. Several of these staff could be shared between units in the same region. The shortage of surgeons and limited theatre times to perform the relevant procedures is a major factor compromising patient care; delays in fashioning vascular fistulas mean that prolonged temporary vascular access is required, resulting in severe morbidity and, not infrequently, in preventable deaths.
Dialysis
Dialysis is expensive, and is conservatively estimated to cost approximately ZAR200 000 per annum per patient. Despite the fact that haemodialysis requires considerably more infrastructure and staff, the cost differential between haemodialysis and peritoneal dialysis is minimal and favours haemo-dialysis as the cheaper option.[10] The high costs of peritoneal dialysis fluid needs to be interrogated, considering that such fluids are locally produced and are less expensive in countries that use locally sourced products.[10]
The summit proposed short- and longterm solutions to improve costs. Developing the new staffing model alluded to above could be an important long-term solution. Other measures that could be instituted almost immediately include tendering for items at a national level to benefit from economies of scale, minimising hospital admissions and stay, ensuring quality dialysis and patient care, and the appropriate use of pharmaceuticals. The summit was cognisant of the fact that a large proportion of our patients are based in rural areas, making ambulatory care difficult. The summit also recommended a 'peritoneal dialysis first' strategy and infrastructure to support this approach. Several countries have instituted incentives to promote peritoneal dialysis, including reducing import duties.[10] Greater use of existing dialysis facilities, which are generally adequate, was also recommended to allow greater numbers of patients access to treatment; this could be achieved in an incremental fashion. Since dialysis machines can be used as often as required, the cost of disposables and lack of personnel are the main limitations to increasing the use of facilities in the public sector. Negotiations with the private sector may allow patients access to dialysis in regions where state facilities are lacking, without a major outlay by government.
Timely referrals of patients with CKD will improve assessment of patients, improve preparation for RRT and obviate the need for acute dialysis (which, in SA, is arguably how the majority of patients present, only to have the diagnosis of CKD confirmed subsequently). Late presentations add to costs in several ways: prolonged hospitalisation, need for temporary vascular access and more intensive dialysis. Besides the significant economic impact, late and ultra-late presentations are associated with poorer patient outcomes, and are potentially avoidable.[11] The ideal of pre-emptive kidney transplantation would reduce costs and improve patient outcomes, but remains an elusive goal; globally only 5% of CKD patients receive transplants without prior dialysis.[12] Although the summit recognised that while healthcare funders bear the brunt of the treatment costs, the economic and psychosocial costs to the patient and his/her family are not insubstantial. The impact of the disease on the patient's lifestyle and ability to seek employment and earn has a direct influence on treatment choices and compliance.
Transplantation
There is no shortage of potential organ donors in SA, as a visit to any busy trauma unit will confirm - translating these into actual donors is where our challenge lies. The current transplant rate of 4.7 pmp in SA is woefully inadequate to meet needs and below the transplant rate of other middle-income countries.[2] The declining number of kidney transplants is the result of declining numbers of donations from deceased donors. The summit has recommended that deceased donation be prioritised. There are several models of organ donation, of which the Spanish and Croatian models are the most effective at increasing deceased donor transplantation; the former has been successfully employed across a diverse range of countries. The successful models have in common an integrated approach including legislative changes, centralisation of authority, employment of transplant co-ordinators responsible for organ recovery, reimbursement of donor hospitals and public awareness campaigns.[13,14] Although countries with an opt-out system have 25 -30% more donations than countries with required consent, in the integrated models - that incorporate opt-out systems - the benefits are of lesser importance. The yield of organs with the integrated models reduces, or may obviate, the need for non-heart-beating and extended criteria donors.[13] The current reliance on living donor transplants is of some concern, as there is recent evidence that in the long term, altruistic kidney donors may suffer some ill health.[15] New deceased donor concepts that bear consideration include those of reciprocity and prioritisation, where persons who previously registered as donors are prioritised should they require a kidney. This has led to a dramatically significant increase in donors and transplants in Israel.[16] The introduction of the Spanish model in Latin America was less successful, with failure ascribed to scarcity of resources and, more importantly, the lack of political will.[17] The importance of the involvement of our NDoH in ensuring the success of such a programme therefore cannot be over-emphasised. Controversy surrounds the use of incentives for organ donation. Notably, the World Medical Association, the WHO and the Convention on Human Rights and Biomedicine all support compensation for expenses the living donor may have incurred.[18] The effect of other measures that have been suggested to increase organ donation appears to be limited.
There is an urgent need to improve access to RRT for patients using the public health service in a fair and equitable fashion, and the summit's call for '250 and 25 by 2025!', which alludes to the plan to increase dialysis to 250 pmp and kidney transplantation to 25 pmp by 2025 (from 164 and 4, respectively), needs to become a clarion call!
Reducing the burden of kidney disease
The SA government's National Development Plan - 2030 emphasises prevention of disease, but to a large extent CKD is the end result of a much larger health challenge facing our country. Diseases such as hypertension, diabetes mellitus, and to a lesser extent infections and acute kidney injury lead to CKD. These diseases need to be appropriately managed to reduce the risk of the development of CKD. Diabetes mellitus will increase by 88% between 2012 and 2030 in sub-Saharan Africa, and hypertension by 70% between 2008 and 2025.[19] The diabetes mellitus epidemic is driven by our sedentary lifestyle and poor nutritional choices that contribute to one of the largest health challenges facing our society - obesity. Almost 70% of SA women are overweight or obese; of even greater concern is that over 25% of girls are also overweight or obese.[20] Managing the lifestyle diseases will ultimately have a beneficial effect on CKD. Community-based screening for CKD is not cost-effective, but high-risk patients - mainly those with diabetes and hypertension - would benefit from strategies that reduce the risk of developing, and retard progression to, ESKD. Such an initiative could be driven by health workers in primary care.
In closing
Each week, the equivalent of two planeloads of SA lives are lost because of lack of access to RRT. This appalling situation is steadily getting worse. Appeals from clinicians to health authorities for greater access to treatment have been met with the usual refrain that resources are insufficient - an explanation that is particularly disappointing in face of funds being diverted from the national fiscus to a range of expenditures that benefit the citizenry not at all.
We are presenting the NDoH with a well-considered and workable blueprint for addressing the crisis of CKD and its treatment. As patient advocates who have to manage the crisis, we challenge the government to work with us to improve care for patients with CKD. Every time one of our patients dies, it is an indictment on us all.
Acknowledgements. We gratefully acknowledge the valuable contributions made by all the participants of the Ministerial NDoH Summit, as well as those of Prof. Melvyn Freeman and his team (for organising the event), Prof. Yosuf Veriava for his leadership in the process, and Prof. Charles Swanepoel for his critical review of this article.
References
1. Stanifer JW, Jing B, Tolan S, et al. The epidemiology of chronic kidney disease in sub-Saharan Africa: A systematic review and meta-analysis. Lancet Global Health 2014;2(3):174-181.[http://dx.doi.org/10.1016/S2214-109X(14)70002-6] [ Links ]
2. Davids MR, Marais N, Jacobs JC. South African Renal Registry Report 2012. Cape Town: South African Renal Society, 2014. [ Links ]
3. Moosa MR, Kidd M. The dangers of rationing dialysis treatment: The dilemma facing a developing country. Kidney Int 2006;70(6):1107-1114. [http://dx.doi.org/10.1038/sj.ki.5001750] [ Links ]
4. Kevany S, Benatar SR, Fleischer T. Improving resource allocation decisions for health and HIV programmes in South Africa: Bioethical, cost-effectiveness and health diplomacy considerations. Global Public Health 2013;8(5):570-587. [http://dx.doi.org/10.1080/17441692.2013.790461] [ Links ]
5. Crisp N, Chen L. Global supply of health professionals. N Engl J Med 2014;370(10):950-957. [http://dx.doi.org/10.1056/NEJMra1111610] [ Links ]
6. Naicker S. Burden of end-stage renal disease in sub-Saharan Africa. Clin Nephrol 2010;74(Suppl 1):S13-S16. [http://dx.doi.org/10.5414/CNP74S013] [ Links ]
7. Yoder LA, Xin W, Norris KC, Yan G. Patient care staffing levels and facility characteristics in U.S. hemodialysis facilities. Am J Kidney Dis 2013;62(6):1130-1140. [http://dx.doi.org/10.1053/j.ajkd.2013.05.007] [ Links ]
8. Thomas-Hawkins C, Flynn L, Clarke SP. Relationships between registered nurse staffing, processes of nursing care, and nurse-reported patient outcomes in chronic hemodialysis units. Nephrol Nurs J 2008;35(2):123-131. [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2845981] [ Links ]
9. Outpatientrenaldialysisfacilities:FactSheet.https://publichealthoregongov/ProviderPartnerResources/HealthcareProvidersFacilities/HealthcareHealthCareRegulationQualityImprovement/Documents/ESRD_RulesFactSheet_2012 (accessed 15 July 2015). [ Links ]
10. Karopadi AN, Mason G, Rettore E, Ronco C. Cost of peritoneal dialysis and haemodialysis across the world. Nephrol Dial Transplant 2013;28(10):2553-2569. [http://dx.doi.org/10.1093/ndt/gft214] [ Links ]
11. Udayaraj UP, Haynes R, Winearls CG. Late presentation of patients with end-stage renal disease for renal replacement therapy - is it always avoidable? Nephrol Dial Transplant 2011;26(11):3646-3651. [http://dx.doi.org/10.1093/ndt/gfr164] [ Links ]
12. Huang Y, Samaniego M. Preemptive kidney transplantation: Has it come of age? Nephrol Ther 2012;8(6):428-432. [http://dx.doi.org/10.1016/j.nephro.2012.06.004] [ Links ]
13. Zivcic-Cosic S, Busic M, Zupan Z, et al. Development of the Croatian model of organ donation and transplantation. Croat Med J 2013;54(1):65-70. [http://dx.doi.org/10.3325/cmj.2013.54.65] [ Links ]
14. Matesanz R, Miranda B. A decade of continuous improvement in cadaveric organ donation: The Spanish model. J Nephrol 2002;15(1):22-28. [ Links ]
15. Mjoen G, Hallan S, Hartmann A, et al. Long-term risks for kidney donors. Kidney Int 2014;86(1):162-167. [http://dx.doi.org/10.1038/ki.2013.460] [ Links ]
16. Cronin AJ. Points mean prizes: Priority points, preferential status and directed organ donation in Israel. Isr J Health Policy Res 2014;3(1):8. [http://dx.doi.org/10.1186/2045-4015-3-8] [ Links ]
17. Matesanz R. Factors influencing the adaptation of the Spanish model of organ donation. Transplant International 2003;16(10):736-741. [http://dx.doi.org/10.1111/j.1432-2277.2003.tb00233.x] [ Links ]
18. Dalal AR. Philosophy of organ donation: Review of ethical facets. World J Transplant 2015;5(2):44-51. [http://dx.doi.org/10.5500/wjt.v5.i2.44] [ Links ]
19. International Diabetes Federation. IDF Diabetes Atlas. 6th ed. Brussels, Belgium: International Diabetes Federation, 2013. [ Links ]
20. Baleta A, Mitchell F. Country in Focus: Diabetes and obesity in South Africa. Lancet Diabetes Endocrinol 2014;2(9):687-688. [http://dx.doi.org/10.1016/S2213-8587(14)70091-9] [ Links ]
21. US Renal Data System. USRDS 2014 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases, 2014. [ Links ]
22. United Nations Development Programme, Human Development Reports. GNI per capita in PPP terms (constant 2011 PPP$). http://hdr.undp.org/en/content/gni-capita-ppp-terms-constant-2011-ppp (accessed 29 July 2015). [ Links ]
 Correspondence:
Correspondence:
M R Moosa
rmm@sun.ac.za
Accepted 28 September 2015.














