Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.106 no.1 Pretoria Jan. 2016
http://dx.doi.org/10.7196/SAMJ.2016.V106I1.9934
IN PRACTICE
DIAGNOSIS
Diagnosis of iron deficiency anaemia in hospital patients: Use of the reticulocyte haemoglobin content to differentiate iron deficiency anaemia from anaemia of chronic disease
E SchapkaitzI; S BuldeoII; J N MahlanguIII
IHaematologist in the Charlotte Maxeke Johannesburg Academic Hospital National Health Laboratory Service (NHLS) Complex and the Department of Molecular Medicine and Haematology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIClinical pathologist at the NHLS and the Department of Haematology, School of Clinical Medicine, College of Health Sciences, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
IIIHead of the School of Pathology, Faculty of Health Sciences, University of the Witwatersrand, Head of the Diagnostic Section and Quality Management in the Department of Molecular Medicine and Haematology, NHLS, and Director of the Bleeding Disorders Unit at Charlotte Maxeke Johannesburg Academic Hospital
ABSTRACT
The diagnosis of iron deficiency anaemia in hospital patients with chronic infections and inflammation presents a challenge. Recently laboratory tests such as the reticulocyte haemoglobin content, which are independent of infection and inflammation, have become available for routine diagnostic use.
Iron deficiency is one of the most common nutritional problems in the world and the leading cause of anaemia in children and pregnant women.[1] In South Africa (SA), iron deficiency and anaemia constitute a significant disease burden owing to chronic helminth infections and a predominantly cereal-based diet.[2]
Iron deficiency is treatable. Successful management of iron deficiency anaemia (IDA) requires accurate diagnosis followed by investigation of the underlying cause of iron loss and treatment with iron supplements. Accurate diagnosis demands differentiation of IDA from the anaemia of chronic disease (ACD), also referred to as anaemia of inflammation. In hospital patients, chronic infection(s) and inflammation often coexist with iron deficiency. In SA there is a high burden of chronic infections such as tuberculosis and HIV.[3] Anaemia has been reported in up to 95% of HIV patients, reflecting cytokine dysregulation, drug therapy, presence of infection, presence of malignancy and/or nutritional deficiencies.[4]
The distinction between concomitant iron deficiency and ACD is often difficult. Characteristically ACD is a mild to moderate anaemia (haemoglobin concentration 8.0 - 9.5 g/dL), which is normocytic and normochromic. However, ACD may also be microcytic and/or hypochromic. It is then important to distinguish it from true IDA, so that appropriate supplementaion can be administered.
Laboratory investigations
Bone marrow (BM) biopsy and iron staining is considered the gold standard test for the diagnosis of iron deficiency. However, BM biopsy is an invasive procedure and is no longer considered the standard of care for assessment of iron stores.[5] In everyday clinical practice, IDA and ACD are traditionally differentiated by assessment of iron studies, which include serum iron, transferrin, transferrin saturation and ferritin (Table 1).[6] A low ferritin level is highly sensitive for the diagnosis of IDA, but ferritin is also an acute- phase reactant showing an increase in the presence of infection or inflammation when iron is sequestered in reticuloendothelial system macrophages. A normal ferritin level therefore does not exclude accompanying IDA.[7] This is evident by the reduced ferritin sensitivity of 46.4% at the clinically recommended cut-off of 30 μg/L in hospital patients.[3]
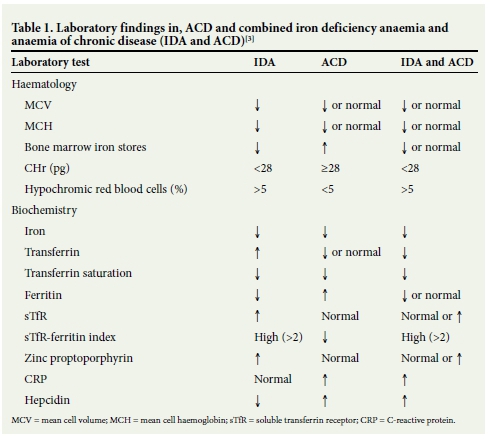
More recently, automated analysers can perform tests that are reported to be independent of infection and inflammation. These include biochemical parameters, namely zinc protoporphyrin and soluble transferrin receptor, as well as haematological parameters, namely the percentage of hypochromic red blood cells, and reticulocyte parameters such as the reticulocyte haemoglobin content (CHr). The advantage of measuring the haemoglobin of the reticulocyte is that the reticulocyte has a shorter lifespan (1 - 2 days) than the red cell. CHr therefore provides an early indicator of iron deficiency.[8]
The reticulocyte haemoglobin content
The CHr is a measure of the product of the reticulocyte haemoglobin concentration and the mean cell volume. Several recent studies[8-11] have confirmed the diagnostic performance of the CHr, which is routinely available.
At the National Health Laboratory Service Haematology Lab- oratory at Charlotte Maxeke Johannesburg Academic Hospital, SA, we performed a prospective study in 74 hospital patients in order to compare the accuracy of the CHr with that of standard haematological and biochemical tests for the diagnosis of IDA using the BM iron stain as the reference.[3] In this study, a CHr of >28 pg reliably distinguished IDA from ACD with a sensitivity of 75.86% and a specificity of 84.10%. CHr is therefore a good discriminatorof IDA.
However, the diagnostic CHr cut-off values vary according to the study population and diagnostic inclusion criteria, emphasising the importance of determining the CHr cut-off for each specific patient population.
In a study by Karlsson,[10] a higher CHr cut-off of 30.5 pg, corresponding to a sensitivity of 93% and a specificity of 69%, was shown to be indicative of IDA in 54 elderly patients. Studies performed in elderly patients have reported a higher mean cell volume (MCV) and mean cell haemoglobin (MCH) in the IDA and ACD groups.[10,12]
A study performed at Pelonomi Regional Hospital, SA, in 100 infants and children aged 6 months - 6 years showed that the optimal CHr cut-off for the diagnosis of iron deficiency was 29 pg. This corresponded to a sensitivity of 86% and a specificity of 50%, using a transferrin saturation of <25% as the diagnostic criterion for iron deficiency.[13]
The CHr has been compared with standard haematological and biochemical tests for the diagnosis of IDA. We found that the sensi- tivity of the CHr was not superior to the MCH parameter or the transferrin saturation, which is similar to the results of two other studies performed in hospital patients in which the authors also concluded that the CHr does not perform better than standard tests for IDA.[10,12] Further studies are required in order to identify an improved marker of iron deficiency in hospital patients compared with standard biochemical and haematological tests.
The CHr test has several advantages. It is a simple and cost-effective test. The current diagnostic panel for IDA, which includes a full blood count (FBC), peripheral smear review and iron studies, costs approximately ZAR610.00. A test panel based on the haematological parameters of FBC and CHr (as part of the reticulocyte count) costs approximately ZAR220.00. The CHr can be performed on 1 - 1.5 mL of blood in a single EDTA tube and in children a finger prick would produce an adequate sample, eliminating the need for additional tubes for the biochemical parameters.
The CHr test does have some obvious current limitations for routine use in that it can only be measured by ADVIA haematology analysers (Siemens Diagnostics, USA), the availability of which is laboratory specific. However, more recently reticulocyte parameters (Ret-He and Ret-Y) on the Sysmex haematology analyser (Sysmex Corporation, Japan) have shown good agreement with the CHr.[12,14] Also, since the CHr is calculated from the reticulocyte MCV, patients with haemoglobinopathy associated with microcytosis will have a falsely low CHr, while patients with megaloblastic anaemia or macrocytic indices (MCV >100 fl), including some patients on antiretroviral therapy, will have a falsely elevated CHr.[15] For the clinician, it is important to interpret the CHr in the context of the patient's other clinical and laboratory investigations including red cell indices, vitamin B12, folate levels and findings on haemoglobin electrophoresis.
Conclusion
The diagnostic distinction between IDA, ACD and the combined state of IDA and ACD in hospital patients with chronic infections and inflammation can be difficult with standard biochemical and haemato- logical tests. Although the CHr test is not superior to standard tests for IDA, it is a simple and cost-effective alternative to biochemical and haematological parameters for the diagnosis of IDA in hospital patients. It is recommended that in hospital patients with anaemia a C-reative protein (CRP), or other biochemical markers of inflammation, and a CHr be added to the initial FBC assessment. If the CRP is elevated, IDA can be diagnosed in patients with coexistent ACD in the presence of a CHr <28 pg and hypochromic red cell indices.
REFERENCES
1. Stoltzfus RJ. Iron deficiency: Global prevalence and consequences. Food Nutr Bull 2003;24(4 Suppl):S99-S103. [ Links ]
2. Nojilana B, Norman R, Dhansay MA, Labadarios D, van Stuijvenberg ME, Bradshaw D. Estimating the burden of disease attributable to iron deficiency anaemia in South Africa in 2000. S Afr Med J 2007;97(8):741-746. [ Links ]
3. Schapkaitz E, Mahlangu JN, Buldeo S. Evaluation of the reticulocyte haemoglobin content as a screening test for iron deficiency anaemia in hospital patients. Presented at the Laboratory Medicine Congress, Cape Town, South Africa, 28-31 July 2013. Poster presentation. [ Links ]
4. Belperio PS, Rhew DC. Prevalence and outcomes of anemia in individuals with human immunodeficiency virus: A systematic review of the literature. Am J Med 2004;116(Suppl 7A):27S-43S. [http://dxdoi.org/10.1016/j.amjmed.2003.12.010] [ Links ]
5. Barron BA, Hoyer JD, Tefferi A. A bone marrow report of absent stainable iron is not diagnostic of iron deficiency. Ann Hematol 2001;80(3):166-169. [http://dx.doi.org/10.1007/s002770000261] [ Links ]
6. Thomas C, Thomas L. Biochemical markers and hematologic indices in the diagnosis of functional iron deficiency. Clin Chem 2002;48(7):1066-1076. [ Links ]
7. Munoz M, Garcia-Erce JA, Remacha AF. Disorders of iron metabolism. Part 1: Molecular basis of iron homoeostasis. J Clin Pathol 2011;64(4):281-286. [http://dx.doi.org/10.1136/jcp.2010.079046] [ Links ]
8. Mast AE, Blinder MA, Lu Q, Flax S, Dietzen DJ. Clinical utility of the reticulocyte hemoglobin content in the diagnosis of iron deficiency. Blood 2002;99(4):1489-1491. [http://dx.doi.org//10.1182/blood.V99.4.1489] [ Links ]
9. Ullrich C, Wu A, Armsby C, et al Screening healthy infants for iron deficiency using reticulocyte hemoglobin content. JAMA 2005;294(8):924-930. [http://dx.doi.org/10.1001/jama.294.8.924] [ Links ]
10. Karlsson T. Comparative evaluation of the reticulocyte hemoglobin content assay when screening for iron deficiency in elderly anemic patients. Anemia 2011;2011:925907. [http://dx.doi.org/10.1155/2011/925907] [ Links ]
11. Brugnara C, Zurakowski D, DiCanzio J, Boyd T, Platt O. Reticulocyte hemoglobin content to diagnose iron deficiency in children. JAMA 1999;281(23):2225-2230. [http://dx.doi.org/10.1001/jama.281.23.2225] [ Links ]
12. Joosten E, Lioen P, Brusselmans C, Indevuyst C, Boeckx N. Is analysis of the reticulocyte haemoglobin equivalent a useful test for the diagnosis of iron deficiency anaemia in geriatric patients? Eur J Intern Med 2013;24(1):636-6 [http://dx.doi.org/10.1016/j.ejim.2012.09.001] [ Links ]
13. Swart PDR, Rautenbach K, Raubenheimer JE. Reticulocyte haemoglobin content as a diagnostic tool for iron deficiency and iron-deficiency anaemia in ill infants and children. S Afr J Child Health 2014;8):23-27. [http://dx.doi.org/10.7196/SAJCH.645] [ Links ]
14. Brugnara C, Schiller B, Moran J. Reticulocyte hemoglobin equivalent (Ret He) and assessment of iron-deficient states. Clin Lab Haematol 2006;28(5):303-308. [http://dx.doi.org/10.1111/j.1365-2257.2006.00812.x] [ Links ]
15. d'Onofrio G, Chirillo R, Zini G, Caenaro G, Tommasi M, Micciulli G. Simultaneous measurement of reticulocyte and red blood cell indices in healthy subjects and patients with microcytic and macrocytic anemia. Blood 1995;85(3):818-823. [ Links ]
 Correspondence:
Correspondence:
E Schapkaitz
elise.schapkaitz@nhls.ac.za
Accepted 14 October 2015.














