Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SAMJ: South African Medical Journal
versión On-line ISSN 2078-5135
versión impresa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.105 no.12 Pretoria dic. 2015
http://dx.doi.org/10.7196/samj.2015.v105i12.9926
RESEARCH
Molecular characterisation and epidemiological investigation of an outbreak of blaOXA-181carbapenemase-producing isolates of Klebsiella pneumoniae in South Africa
R K JacobsonI, II, III,*; M R ManesenIV, V, VI, *; C MoodleyVII, VIII, IX; M SmithX; S G WilliamsXI, XII; M P NicolXIII, XIV, XV; C M BamfordXVI, XVII, XVIII
IMSc, Centre for Opportunistic, Tropical and Hospital Infections, National Institute for Communicable Diseases, Groote Schuur Hospital, Cape Town, South Africa
IIMSc, National Health Laboratory Service, Groote Schuur Hospital, Cape Town, South Africa
IIIMSc, Division of Medical Microbiology, University of Cape Town, South Africa
IVMPH, South African Field Epidemiology Training Programme, National Institute for Communicable Diseases, Sandringham, Johannesburg, South Africa
VMPH, School of Health Systems and Public Health, University of Pretoria, South Africa
VIMPH, Centers for Disease Control and Prevention, Atlanta, Georgia, USA
VIIPhD, Centre for Opportunistic, Tropical and Hospital Infections, National Institute for Communicable Diseases, Groote Schuur Hospital, Cape Town, South Africa
VIIIPhD,National Health Laboratory Service, Groote Schuur Hospital, Cape Town, South Africa
IXPhD, Division of Medical Microbiology, University of Cape Town, South Africa
XMSc Centre for Opportunistic, Tropical and Hospital Infections, National Institute for Communicable Diseases, Groote Schuur Hospital, Cape Town, South Africa
XIMD, South African Field Epidemiology Training Programme, National Institute for Communicable Diseases, Sandringham, Johannesburg, South Africa
XIIMD, Centers for Disease Control and Prevention, Atlanta, Georgia, USA
XIIIMB BCh, MMed (Med Micro), DTM&H, FCPath (Micro), PhD, Centre for Opportunistic, Tropical and Hospital Infections, National Institute for Communicable Diseases, Groote Schuur Hospital, Cape Town, South Africa
XIVMB BCh, MMed (Med Micro), DTM&H, FCPath (Micro), PhD, National Health Laboratory Service, Groote Schuur Hospital, Cape Town, South Africa
XVMB BCh, MMed (Med Micro), DTM&H, FCPath (Micro), PhD, Division of Medical Microbiology, University of Cape Town, South Africa
XVIMB ChB, MMed (Med Micro) FCPath (Micro),Centre for Opportunistic, Tropical and Hospital Infections, National Institute for Communicable Diseases, Groote Schuur Hospital, Cape Town, South Africa
XVIIMB ChB, MMed (Med Micro) FCPath (Micro), National Health Laboratory Service, Groote Schuur Hospital, Cape Town, South Africa
XVIIIMB ChB, MMed (Med Micro) FCPath (Micro), Division of Medical Microbiology, University of Cape Town, South Africa
ABSTRACT
BACKGROUND: Klebsiella pneumoniae is an opportunistic pathogen often associated with nosocomial infections. A suspected outbreak of K. pneumoniae isolates, exhibiting reduced susceptibility to carbapenem antibiotics, was detected during the month of May 2012 among patients admitted to a haematology unit of a tertiary academic hospital in Cape Town, South Africa (SA).
OBJECTIVES: An investigation was done to determine possible epidemiological links between the case patients and to describe the mechanisms of carbapenem resistance of these bacterial isolates.
METHODS: Relevant demographic, clinical and laboratory information was extracted from hospital records and an observational review of infection prevention and control practices in the affected unit was performed. Antimicrobial susceptibility testing including phenotypic testing and genotypic detection of the most commonly described carbapenemase genes was done. The phylogenetic relationship of all isolates containing the blaOXA-181 carbapenemase gene was determined by pulsed-field gel electrophoresis (PFGE) and multilocus sequence typing.
RESULTS: Polymerase chain reaction analysis identified a total of seven blaOXA-181-positive, carbapenem-resistant K. pneumoniae isolates obtained from seven patients, all from a single unit. These isolates were indistinguishable using PFGE analysis and belonged to sequence type ST-14. No other carbapenemase enzymes were detected.
CONCLUSION: This is the first documented laboratory-confirmed outbreak of OXA-181-producing K. pneumoniae in SA, and highlights the importance of enforcing strict adherence to infection control procedures and the need for ongoing surveillance of antibiotic-resistant pathogens in local hospitals.
Patients infected with multidrug-resistant Entero-bacteriaceae were, until recently and with relative success, treated with carbapenems, a class of broad-spectrum β-lactam antibiotics. Of great concern is the over-use of carbapenems in hospitals for treating Gram-negative bacterial infections, which is contributing to the increasing emergence of carbapenem resistance.[1]
Carbapenem resistance in Klebsiella spp. is mainly attributable to the acquisition of carbapenem-hydrolysing β-lactamase enzymes. The Ambler classification scheme is used to separate these enzymes into classes A, B or D.[3] The OXA-48-like subgroup, of which OXA-181 is a member, are Ambler class D carbapenemases.[3] OXA-48 was first described in the environmental bacterium Shewanella spp., and more recently there have been several reports of this enzyme, mainly in K pneumoniae isolates, from countries in the Middle East, Europe, and northern and southern Africa.[4-7] OXA-181, a variant of OXA-48, was first identified in K. pneumoniae isolated from a Tanzanian patient.[7] It differs by just four amino acid substitutions in the active site, yet still confers the same levels of enzymatic hydrolysis of carbapenems as OXA-48.[7] Several international studies have identified OXA-181-producing Enterobacteriaceae in the UK, the Sultanate of Oman, the Netherlands, Senegal and New Zealand.[7-12] The genetic location of blaOXA-181is typically on a Tn2013 transposon, immediately downstream of the insertion sequence ISEcplB, carried on a 7.6-Kb, ColE-type plasmid.[7]
The prevalence of nosocomial infections in African countries has been under-appreciated in the past, and while such outbreaks occurring internationally are frequently reported in the literature, relatively few of these reports originate from developing countries.[13] However, even in lower-income countries, outbreak reports are necessary to increase awareness in the local medical community and highlight the need for enforced and effective infection prevention and control (IPAC) measures to limit the occurrence and transmission of antibiotic-resistant pathogens. OXA-48-like carbapenem-resistant Enterobacteriaceae are endemic worldwide but had not previously been observed in our hospital. A subsequent report indicates that such organisms had recently been detected in other local institutions.[6] This outbreak report serves as the first description and characterisation of a single outbreak of carbapenem-resistant K. pneumoniae in a haematology unit of a local hospital in Cape Town, South Africa (SA), during the month of May 2012.
Objectives
Following the identification of a possible outbreak of carbapenem-resistant Klebsiella spp. in a haematology unit in Cape Town, an epidemiological investigation was done to describe the extent of the outbreak, ascertain possible transmission routes and identify opportunities to prevent further transmission. Laboratory studies were carried out to characterise the molecular basis for carbapenem resistance and the molecular epidemiology of these isolates.
Methods
This was a prospective study, implemented informally in response to the detection on 12 May 2012 of carbapenem-resistant Klebsiella spp. from two patients in the haematology intensive care unit (ICU) of a 900-bed tertiary care referral hospital in Cape Town.
The haematology ICU is utilised by high-risk haematology patients, either with haematological malignancies or undergoing stem cell transplants, typically when neutro-penic. Patients from both the public hospital and the adjacent private hospital are admitted and nursed in 12 patient isolation rooms by staff from the public or private hospitals, respectively. However, both teams of staff share communal work areas within the unit, including the nurses' station, the sluice room and staff rooms. The unit is supported by infection control staff from both private and public hospitals.
Following the identification of carbapenem-resistant Klebsiella, an immediate and intensive outbreak response was implemented. Infection control measures were intensified, patient movement in and out of the affected unit was halted, and 2 weeks later it was closed for decontamination, maintenance and repairs.
A policy for weekly stool or rectal swab surveillance for carbapenem-resistant Entero-bacteriaceae for all haematology and oncology inpatients (in either the ICU or the associated wards) was already in place prior to May 2012. Laboratory records were checked for any previous carbapenem-resistant Enterobacteriaceae isolates from both clinical and surveillance specimens. Surveillance was intensified, and stool samples were also obtained from staff members to screen for possible gut carriage. Environmental sampling was not conducted as a previous outbreak suggested very limited benefit.[14]
For the outbreak a case was defined as a patient with carbapenem-resistant Klebsiella isolated from a clinical specimen (provider requested) or surveillance (routine screening) culture from the shared haematology ICU from 1 to 31 May 2012. A retrospective review of hospital records was performed for each case patient admitted before 31 May 2012. Demographic, clinical, and laboratory information was abstracted from case patient charts, with all data being collected in the same manner as in the outbreak reported by Marchaim et al.[15]in 2011. Details of the eight patients included in the outbreak are provided in the 'Results' section.
Ethical considerations
As this study was conducted as an outbreak investigation for IPAC purposes, informed consent of patients was not obtained on the grounds that specimen collection was based on routine clinical practice and infection control requirements. The investigation was approved by the Human Research Ethics Committee of the University of Cape Town (Ref: 534/2012).
Bacterial identification and genotypic typing
All stool specimens or rectal swabs were plated onto MacConkey agar containing 4 μg/mL gentamicin. The resulting colonies were subsequently identified using the Vitek 2 system (GN and N133 card, bioMérieux, France) and their antibiotic susceptibility profiles determined (Vitek 2). All isolates with reduced susceptibility to the carbapenems were screened for phenotypic carbapenemase activity using the modified Hodge test. The minimum inhibitory concentrations of carbapenems available in this hospital (ertapenem, imipenem and meropenem) were determined using the E-test method (bioMérieux, France). All tests were done and interpreted according to the Clinical Laboratory Standards Institute criteria (CLSI-2012).[16]
Conventional polymerase chain reaction (PCR) assays were performed using, as template, either total cellular DNA extracted from cultured isolates using the ZR-96 Fungal/ Bacterial DNA kit (Zymo Research, Inqaba, South Africa), or total DNA extracted from stool and rectal swab specimens using either the QiaSymphony (QIAGEN, Germany) or ZR fecal DNA Microprep kit (Zymo Research, Inqaba, South Africa). All extractions were carried out as per the manufacturer's instructions. PCR experiments were performed using PCR primers designed to target internal fragments of the current most clinically significant carbapenemase genes (blaNDM, blaVIM, blaSPM, biaIMp, blaKpc, blaGES and blaoxA-48-like) (Table 1).
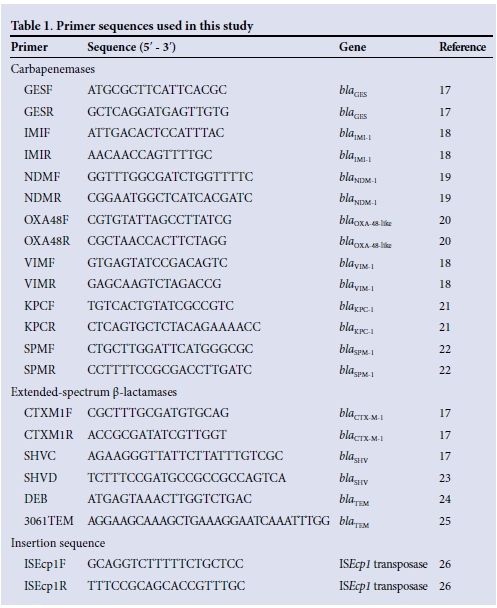
The resulting PCR amplicons were purified (QIAquick pCR purification kit, QIAGEN, Germany) and then sequenced. To test for the presence of extended-spectrum ß-lactamase (ESBL) (Table 1)[17-26] genes blaCTX-M, blaSHVand blaTEM, conventional pCR assays using primers designed to target internal fragments of these genes were performed and any resulting pCR amplicons of the expected size were sequenced.
To describe the genomic arrangement of the identified blaOXA-181 genes in positive isolates in relation to the ISEcplB gene, which had previously been shown to be associated with the OXA-181 gene,[7] conventional PCR was carried out using primers designed to amplify an internal fragment of the insertion element, ISEcp1B, and an internal portion of the OXA-181 gene (Table 1). The resulting PCR amplicons were purified (QIAquick PCR purification kit) and then sequenced to confirm sequence identity.
The relatedness of the Klebsiella isolates was investigated using pulsed-field gel electro-phoresis (PFGE) according to a previously published protocol with minor changes.[27] Total genomic DNA was digested, in situ, with XbaI (New England Biolabs Inc., UK) and the resulting DNA fragments were separated by electrophoresis using a1% w/v, 0.5 χ TBE-agarose gel in a CHEF-DRII GeneNavigator apparatus (GE Healthcare, USA) with a ramped pulse time of 5 - 60 seconds, over 21 hours, at a constant voltage of 200 V in 0.5 χ TBE buffer maintained at 14°C. The resulting restriction profiles were analysed using GelCompar II version 5.1 software (Applied Maths, Belgium). A dendrogram indicating the UPGMA clustering between the isolates was created using the Dice similarity coefficient. The band tolerance and optimisation were set at 1.0% and a similarity threshold of >80% was used to define related clusters.[28]
Multilocus sequence typing (MLST), specific for Klebsiella, was performed using seven housekeeping genes as reference loci (rpoB, gapA, mdh, pgi, phoE, infB, and tonB) according to the method described by Diancourt et al.,[29] with minor amendments. Allele and sequence types were determined using the Institut Pasteur MLST online database for K. pneumoniae.[30]
Results
Outbreak investigation
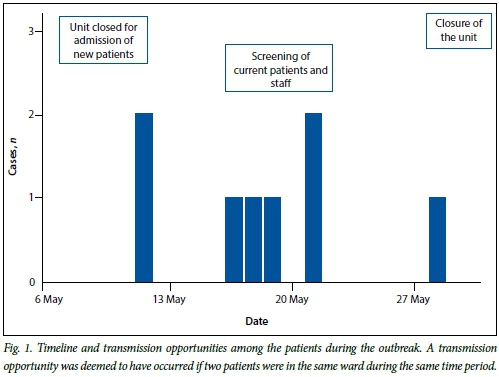
Review of laboratory records showed that no carbapenem-resistant Enterobacteriaceae had been detected prior to 12 May 2012. A total of 340 rectal swabs or stool specimens, from patients and staff members, were collected and screened for carriage of carbapenem-resistant Enterobacteriaceae. A total of eight patients with carbapenem resistant Klebsiella were identified from 12 to 31 May 2012. The eight patients were either infected (blood culture, n=1) or colonised (pus swab, stool, sputum or tracheal aspirate, n=7). No carbapenem-resistant Enterobacteriaceae were identified from the rectal swab specimens submitted by the healthcare workers (HCWs). A timeline of the outbreak is depicted in Fig. 1.
Patient characteristics
A summary of the patients' demographic and clinical information is provided in Table 2. The median age was 48 years (range 30 - 77 years) and half of the patients were male. The median hospitalisation time was 16 days (range 3-29 days). All patients had underlying malignancies, were receiving cancer chemotherapeutic treatment and had been previously exposed to multiple antibiotics, placing them at high risk for acquiring nosocomial infections.
A timeline for detection of carbapenem-resistant Klebsiella isolates indicated that the outbreak occurred over a 2-week period from 12 to 28 May. The floor plan of the affected ICU indicates the placement of 7 of the 8 patients (Fig. 2). Patients 1, 2, 3, 4, 7 and 8 were either placed in the same room as a previous case or in adjacent rooms in close proximity to communal areas, while patient 5 was in close proximity to the doctors' room and a common staff room. Seven of the eight patients were admitted to the shared haematology unit for the duration of their hospital stay. The remaining patient (patient 6), who had a respiratory tract colonisation, was identified in a separate ICU, but could be epidemiologically linked to patient 1 because he was transferred and admitted to the bed adjacent to patient 6. Patient 1 died from a bloodstream infection (6 hours post transfer), and 8 days later a carbapenem-resistant K. pneumoniae was cultured from an endotracheal specimen from patient 6.
Infection control
During the inspection of the IPAC practices in the affected ICU, breaches in contact precaution including lack of use of aprons and gloves by HCWs before entering patient rooms, incorrect disposal of these items and poor hand hygiene were observed. In addition, incorrect signage relating to standard IPAC practices and inadequate signage for patients about contact precautions was also documented.
Microbiological characterisation of Klebsiella isolates
A total of eight carbapenem-resistant Kleb-siella isolates (seven K. pneumoniae and one K. oxytoca) were identified. All eight isolates exhibited a multidrug-resistance phenotype with resistance to ampicillin, co-amoxiclav, cefuroxime, cefoxitin, cefotaxime, cefta-zidime, cefepime, gentamicin, amikacin, tigecycline, co-trimoxazole, nalidixic acid and ciprofloxacin, and were susceptible to colistin only. Carbapenem minimum inhibitory concentration (MIC) patterns are shown in Table 2. The isolates demonstrated variable susceptibility to imipenem with reduced susceptibility to both meropenem (range 8 - >32 mg/L) and ertapenem (all >32 mg/L). The modified Hodge test was positive for all eight isolates.[31]
Conventional PCR for OXA-48 and OXA-48-like genes generated amplicons of the expected size for all eight isolates. Subsequent sequence analysis revealed that all eight PCR positive isolates had 100% homology with blaOXA-181, a genetic variant of blaOXA-48. Similar results were obtained from DNA extracted from the corresponding rectal swabs or stool samples. No amplicons were obtained for the other carbapenemase genes screened: bla^M, blaVM, blaMP, blaKP0blasPM and blaGES All eight isolates were positive on ESBL PCR screening for blaCTX-M, blaTEM and blaSHV (with the exception of K. oxytoca, which did not contain blaSHV), as shown in Table 2.
Genetic arrangement of the blaOXA-181
Using the forward primer targeting IS£cp1B and the reverse primer internal to blaOXA-181, an amplicon of the expected size was obtained, suggesting these genes are associated in all eight isolates.
Phylogenetic analysis
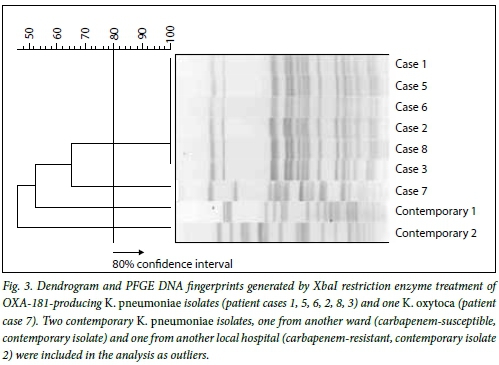
To establish the relatedness of the seven K. pneumoniae isolates, PFGE analysis was carried out. K. pneumoniae isolate 4 was not viable. The resultant PFGE fingerprints of six of the K. pneumoniae isolates, patients 1, 2, 3, 5, 6, and 8, were indistinguishable (100% identity) and were assigned to cluster A (Fig. 3). Two contemporary K. pneumoniae isolates, one from another ward (carbapenem susceptible) and one from another local hospital (carbapenem resistant) were included in the analysis and shown to be unrelated to cluster A. The K. oxytoca isolate from patient 7 was, as expected, genetically distinct from all the K. pneumoniae strains tested. Since the six K. pneumoniae isolates had the same PFGE profile, MLST analysis was only carried out on two representative K. pneumoniae isolates, case numbers 6 and 4. The results were analysed using the Pasteur database and showed that these two strains are both members of sequence type ST14.
Discussion and conclusions
This study describes the first documented nosocomial outbreak of blaOXA-181-producing, carbapenem-resistant K. pneumoniae and K. oxytoca isolated from a local tertiary academic hospital in Cape Town. Resistance to the clinically significant carbapenem antibiotics by enzymatic hydrolysis has been described in Enterobacteriaceae worldwide, and recently the class D β-lactamase, OXA-48 and its variants have been detected more frequently.[4] While the blaOXA-181gene has previously been identified in several clonally unrelated K. pneumoniae isolates from SA, India, Singapore, the Netherlands and New Zealand,[4-12] this is the first report describing an outbreak of blaOXA-181 in SA. To our knowledge this is the first identification of a blaOXA-181-producing K. oxytoca isolate. Although it remains unclear where this organism originated, and since no international travel link could be established for any of the patients, there is evidence to suggest that OXA-181 may have been prevalent in a nearby private sector hospital that could have been a possible source of this organism in the community.[6] The profiles of six of the K. pneumoniae isolates, patients 1, 2, 3, 5, 6, and 8, were indistinguishable and were assigned to cluster A. Two contemporary K. pneumoniae isolates, one from another ward (carbapenem-susceptible) and one from another local hospital (carbapenem-resistant), were included in the analysis and shown to be unrelated to cluster A. PFGE is no longer considered the gold standard for genetic typing because of its lack of discrimination. Therefore, to characterise the genetic background of this clone further, MLST of two representative isolates from cluster A, spanning the defined outbreak period, were selected. MLST indicated that these two isolates belonged to sequence type ST14. K. pneumoniae ST14 has on two previous occasions been associated with ESBLs and not with metallo-ß-lactamases or serine carbapenemases, as was observed in these isolates.[10,11]
All OXA-181-containing isolates in this study were resistant to carbapenems and positive for carbapenemase production by the modified Hodge test. The co-production of OXA-181 with ESBL enzymes and the accumulation of additional ß-lactamase genes in Enterobacteriaceae has been shown to collectively produce an elevated multidrug-resistance phenotype.[4,7,9,10] A probable explanation for the uncharacteristically high carbapenem resistance levels observed with these isolates was the presence of additional ESBL genes. Furthermore, the OXA-181 gene was shown to be linked to the ISEcp 1B element, which has been shown to up-regulate gene expression and allow for gene mobilisation.[7] Further investigations are underway to determine the plasmid types of these isolates to better elucidate reasons for its successful spread.
The IPAC report documented inadequate infection control practices among healthcare staff in the shared haematology unit, and it is possible that this breakdown in infection control practices contributed to the spread of this organism between patients, either directly via the hands of staff or via fomites. It was observed among HCWs that breaches in contact precautions, including the lack of aprons and gloves prior to entering patient rooms, as well as the incorrect disposal of these items, and overall poor hand hygiene could have contributed to the spread of this organism. HCWs were encouraged to submit faecal samples for the screening of gut carriage of carbapenem-resistant Enterobacteriaceae as a possible source of the outbreak. This investigation could not rule out transmission in other communal areas, such as the radiology department or procedure rooms used for implanting medical devices. Further case-control analysis of patient and provider risk factors would have strengthened this investigation. In addition, this study could not clearly identify an index patient, given the inconsistent compliance with surveillance screening policy prior to the outbreak. The following recommendations were made based on the IPAC report from this investigation: stricter adherence to laboratory screening for antibiotic-resistant organisms from routine surveillance rectal swabs of new patients, both on admission and weekly follow-up, as well as the re-education and adherence to IPAC among all staff (temporary and full time). Additionally, improved IPAC signage practices that will increase awareness among hospital staff, the identification of IPAC awareness champions and developing a multidisciplinary team to maintain awareness should be considered. Ultimately, timely identification of the organism, swift unit closure and a reduction in patient movement coupled with strict contact precautions for colonised patients were effective in limiting further transmission of this isolate, as there were no new cases identified after 28 May. However, given the ongoing circulation of patients between local hospitals, and the potential for long-term asymptomatic carriage, it is likely that carbapenem-resistant Klebsiella will be reintroduced in the future. Vulnerable patients with haematological malignancies are likely to manifest infections as a result of multidrug-resistant organisms, including carbapenem-resistant Klebsiella.
In summary, this outbreak demonstrates the importance of active surveillance coupled with rapid and accurate molecular genotypic testing in enabling the detection and subsequent control of carbapenemaseproducing Klebsiella. This work highlights the necessity of not only ensuring that effective infection control measures are in place to identify and limit the spread of such organisms, but also the added value molecular screening offers in confirming an outbreak by determining the molecular epidemiology of the bacterial isolates and the prevalence of resistance genes circulating in our hospitals and communities.
Author contributions. All authors were involved in the conceptualisation of the study. RKJ, CM, MS, CB and MRM collected the data. MRM, MS, SW and CB investigated the outbreak. RKJ, CM, MS, CB, MN, MRM, and SW carried out the data analysis and interpretation. RKJ and MRM drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgements. We acknowledge the National Health Laboratory Service Microbiology Division, Department of Medical Microbiology, University of Cape Town, as well as Sisters Morris, Engelbrecht and Boonzaaier, local infection control sisters and Seymour Williams and Alexander Kallen of the Centers for Disease Control and Prevention, USA. In addition, we acknowledge that the data for the MLST online database for K. pneumoniae are publicly available at http://www.pasteur.fr/mlst.
Funding. This work was supported by the Global Disease and Detection Funding, Centre for Opportunistic Tropical and Hospital Infections, National Institute for Communicable Diseases and the National Health Laboratory Service, Johannesburg.
References
1. Fishman N. Antimicrobial stewardship. Am J Med 2006;119(6A):S53-S61. [http://dx.doi.org/10.1016/jamjmed.2006.04.003] [ Links ]
2. Goff D. Antimicrobial stewardship: Bridging the gap between quality care and cost. Curr Opin Infect Dis 2011;24(Suppl 1):S11-S20. [http://dx.doi.org/10.1097/01.qco.0000393484.17894.05] [ Links ]
3. Hall BG, Barlow M. Revised Ambler classification of beta-lactamases. J Antimicrob Chemother 2005;55(6):1050-1051. [http://dx.doi.org/10.1093/jac/dkil30] [ Links ]
4. Poirel L, Potron A, Nordmann P. OXA-48-like carbapenemases: The phantom menace. J Antimicrob Chemother 2012;67(7):1597-1606. [http://dx.doi.org/10.1093/dks121] [ Links ]
5. Poirel L, Héritier C, Tolün V, Nordmann P. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob Agents Chemother 2004;48(1):15-22. [http://dx.doi.org/10.1128/AAC.48.1.15-22.2004] [ Links ]
6. Brink AJ, Coetzee J, Corcoran C, et al. Emergence of OXA-48 and OXA-181 carbapenemases amongst Enterobacteriaceae in South Africa, and evidence of in vivo selection of colistin resistance as a consequence of selective decontamination of the gastro-intestinal tract. J Clin Microbiol 2013;51(1):369-372. [http://dx.doi.org/10.1128/JCM.02234-12] [ Links ]
7. Potron A, Nordmann P, Lafeuille E, et al. Characterization of OXA-181, a carbapenem-hydrolyzing class D beta-lactamase from Klebsiella pneumoniae. Antimicrob Agents Chemother 2011;55(10):4896-4899. [http://dx.doi.org/10.1128/AAC.00481-11] [ Links ]
8. Dimou V, Dhanji H, Pike R, Livermore DM, Woodford N. Characterization of Enterobacteriaceae producing OXA-48-like carbapenemases in the UK. J Antimicrob Chemother 2012;67(7):1660-1665. [http://dx.doi.org/10.1093/jac/dks124] [ Links ]
9. Dortet L, Poirel L, Al Yaqoubi F, Nordmann P. NDM-1, OXA-48 and OXA-181 carbapenemase- producing Enterobacteriaceae in Sultanate of Oman. Clin Microbiol Infect 2012;18(5):E144-148. [http://dx.doi.org/10.1111/j.1469-0691.2012.03796. [ Links ]
10. Kalpoe JS, Al Naiemi N, Poirel L, Nordmann P. Detection of an Ambler class D OXA-48-type β-lactamase in a Klebsiella pneumoniae strain in The Netherlands. J Med Microbiol 2011;60(5):677-678. [http://dx.doi.org/10.1099/jmm.0.028308-0] [ Links ]
11. Balm MND, Ngan G, Jureen R, et al. OXA-181-producing Klebsiella pneumoniae establishing in Singapore. BMC Infect Dis 2013;13:58. [http://dx.doi.org/10.1186/1471-2334-13-58] [ Links ]
12. Williamson DA, Heffernan H, Sidjabat H, et al Intercontinental transfer of OXA-181-producing Klebsiella pneumoniae into New Zealand. J Antimicrob Chemother 2011;66(12):2888-2890. [http://dx.doi.org/10.1093/jac/dkr396] [ Links ]
13. Manenzhe R, Zar H, Nicol M, Kaba M. The spread of carbapenem producing bacteria: A systematic review. J Antimicrob Chemother 2015;70(1):23-40. [http://dx.doi.org/10.1093/jac/dku356] [ Links ]
14. Madau M, Jacobson R, Minenza N, et al Outbreak of multi-drug resistant Pseudomonas aeruginosa bloodstream infection in the haematology unit of a South African academic hospital. PLoS One 2013;8(3):E55985. [http://dx.doi.org/10.1371/journal.pone.0055985] [ Links ]
15. Marchaim D, Chopra T, Pogue JM, et al. Outbreak of colistin-resistant, carbapenem-resistant Klebsiella pneumoniae in metropolitan Detroit, Michigan. J Antimicrob Chemother 2011;55(2):593-599. [http://dx.doi.org/10.1128/AAC.01020-10] [ Links ]
16. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-second Informational Supplement, M100-S22S21. Wayne, PA: CLSI, 2012. [ Links ]
17. Segal H, Elisha BG. Resistance to beta-lactams, and reduced susceptibility to carbapenems, in clinical isolates of Klebsiella pneumoniae due to interplay between CTX-M-15 and altered outer membrane permeability. South African Journal of Epidemiology and Infection 2006;21(2):41-44. [ Links ]
18. Jacobson RK, Minenza N, Nicol M, Bamford C. VIM-2 metallo-beta-lactamase producing Pseudomonas aeruginosa causing an outbreak in South Africa. J Antimicrob Chemother 2012;67(7):1797-1798. [http://dx.doi.org/10.1093/jac/dks100] [ Links ]
19. Poirel L, Revathi G, Bernabeau S, Nordman P. Detection of NDM-1-producing Klebsiella pneumoniae in Kenya. Antimicrob Agents Chemother 2011;55(2):934-936. [http://dx.doi.org/10.1128/AAC.01247-10] [ Links ]
20. Manesen R, Bamford C, Smith M, et al. Outbreak of carbapenem-resistant Klebsiella species in an academic hospital, Western Cape, May 2012. National Institute for Communicable Diseases Surveillance Bulletin 2012;10(4):82-86. [ Links ]
21. Yigit H, Queenan AM, Anderson GJ, et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC- 1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother 2001;45(4):1151-1161. [http://dx.doi.org/10.1128/AAC.45.4] [ Links ]
22. Poirel L, Magalhaes M, Lopes M, Nordmann P. Molecular analysis of metallo-β lactamase gene blaSPM-1-surrounding sequences from disseminated Pseudomonas aeruginosa isolates in Recife, Brazil. Antimicrob Agents Chemother 2004;48(4):1406-1409. [http://dx.doi.org/10.1128/ AAC.48.4.1406-1409.2004] [ Links ]
23. Perilli M, Dell'Amico E, Segatore B, et al. Molecular characterization of extended spectrum beta- lactamases produced by nosocomial isolates of Enterobacteriaceae from an Italian nationwide survey. J Clin Microbiol 2002;40(2):611-614. [http://dx.doi.org/10.1128/JCM.40.2.611-614.2002] [ Links ]
24. Canica MM, Lu CY, Krishnamoorthy R, Paul GC. Molecular diversity and evolution of blaTEM genes encoding beta-lactamases resistant to clavulanic acid in clinical E. coli. J Mol Evol 1997;44(1):57-65. [ Links ]
25. Nelson EC, Segal H, Elisha BG. Outer membrane protein alterations and blaTEM-1 variants: Their role in β-lactam resistance in Klebsiella pneumoniae. Antimicrob Agents Chemother 2003;52(6):899-903. [http://dx.doi.org/10.1093/jac/dkg486] [ Links ]
26. Poirel L, Decousser J, Nordmann P. Insertion sequence ISEcp1B is involved in expression and mobilization of a bla(CTX-M) beta-lactamase gene. Antimicrob Agents Chemother 2003;47(9):2938-2945. [http://dx.doi.org/10.1128/AAC.47.9.2938-2945.2003] [ Links ]
27. Pulse Net USA. One-day (24-28 h) standardized laboratory protocol for molecular subtyping of Escherichia coli O157:H7, Salmonellaserotypes, Shigella sonnei, and Shigella flexneri by pulsed field gel electrophoresis (PFGE) 2009;(10):1-16. http://www.pulsenetinternational.org/assets/PulseNet/uploads/pfge/PNL05_Ec-Sal-ShigPFGEprotocol.pdf (accessed 6 November 2015). [ Links ]
28. Tenover FC, Arbeit RD, Goering RV, et al Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: Criteria for bacterial strain typing. J Clin Microbiol 1995;33(9):2233-2239. [ Links ]
29. Diancourt L, Passet V, Verhoef J, et al. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J Clin Microbiol 2005;43(8):4178-4182. [http://dx.doi.org/10.128/JCM.43.8.4178-4182.2005] [ Links ]
30. Institut Pasteur. Klebsiella pneumoniae MLST database. http://www.pasteur.fr/mlst (accessed 24 January 2015). [ Links ]
31. Girlich D, Poirel L, Nordmann P. Value of the modified Hodge test for the detection of emerging carbapenemases in Enterobacteriaceae. J Clin Microbiol 2012;50(2):477-479. [http://dx.doi.org/10.1128/JCM.05247-11] [ Links ]
 Correspondence:
Correspondence:
R K Jacobson
rachaeljacobson@gmail.com
Accepted 28 September 2015.
* These authors are co-first authors.














