Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.105 n.10 Pretoria Oct. 2015
http://dx.doi.org/10.7196/SAMJNEW.8329
RESEARCH
Pulmonary tuberculosis in a South African regional emergency centre: Can infection control be improved to lower the risk of nosocomial transmission?
H CaseyI; A SmithII; L ParkerIII; M DipperIV; T GouldV
IMB ChB; NHS Thames Valley and Wessex Leadership Academy, UK
IIMB ChB, MMed (EM), FCEM (SA); Department of Emergency Medicine, George Hospital, Western Cape, South Africa
IIIMB ChB, MRCP;NHS Thames Valley and Wessex Leadership Academy, UK
IVBM;NHS Thames Valley and Wessex Leadership Academy, UK
VMB BCh, FCP (SA), MMed; Internal Medicine Unit, George Hospital, Western Cape, South Africa
ABSTRACT
BACKGROUND: George Regional Hospital (GRH) is a 272-bed regional referral hospital for the Eden and Central Karoo districts, Western Cape Province, South Africa. The perception among emergency centre (EC) staff is that a high burden of tuberculosis (TB) is being diagnosed and that infection control procedures are currently lacking, leading to a high risk of nosocomial transmission.
OBJECTIVES: To establish the burden of pulmonary TB (PTB) presenting to GRH via the EC and audit current infection prevention and control practices, to quantify the risk of transmission of PTB in the EC and to establish whether infection control measures are inadequate.
METHODS: An audit of infection control based on the Centers for Disease Control audit tool for TB, analysis of results, and implementation of new infection control measures including a new standard operating procedure based on a set of triage criteria.
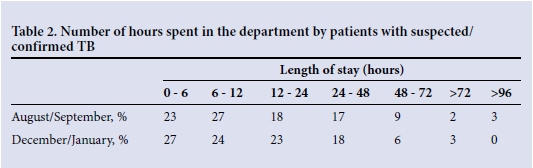
RESULTS: Implementation of new triage criteria and a standard operating procedure led to the longest length of stay of a patient with suspected TB in the EC being reduced by 40% (from 142 hours to 84 hours). The average time between seeing a doctor and leaving the EC for patients with suspected TB was reduced by 20% (from 20 hours 40 minutes to 16 hours 45 minutes.
CONCLUSION: Simple measures implemented in the EC led to a significant reduction in the time patients with suspected or confirmed TB spent in the EC. This should lead to a reduced risk of nosocomial transmission of TB to both staff and patients.
Tuberculosis (TB) is a major burden in South Africa (SA), and prevalence rates in the Western Cape Province are exceptionally high. In 2010, TB was the second leading cause of male deaths after interpersonal violence, with the burden of TB highest in the younger population, contributing to a large number of early deaths.[1] The most significant change in disease burden in recent years is the simultaneous increase in comorbid HIV infection and the rise of multidrug-resistant (MDR) TB. The challenge over the coming years will be to prevent hospitals from becoming sources of nosocomial infections for both healthcare workers and patients.[2]
The Western Cape Government healthcare strategy up to 2030[2] states that 'All acute admitting institutions must have appropriate infection control measures to the extent that they would be comfortable to retain a drug-resistant case for 24 to 72 hours
To be confident in managing acute presentations of pulmonary tuberculosis (PTB) at a facility level, the infection prevention and control (IPC) measures used in the hospital must be of a high standard. IPC measures for use in low-resource settings have been published by both the World Health Organization (WHO) and the US Centers for Disease Control (CDC).[3,4] Infection control is divided into three areas: administrative, environmental, and personal protective equipment. Studies looking at the implementation of infection control in SA at facilities ranging from primary healthcare facilities to specialist centres for MDR TB have found that practice between practitioners and facilities is highly variable.[5-7]
Research carried out in low- and middle-income countries has shown that healthcare workers are at considerable risk of contracting TB in high-risk settings through nosocomial transmission.[8] Studies in SA have shown a high rate of TB among healthcare workers in settings ranging from primary healthcare facilities to tertiary teaching hospitals.[6,9] This includes a high rate of conversion to latent TB infection by medical students and practising healthcare workers.[10]
In high-income settings the emergency centre (EC) has been shown to be a high-risk area owing to presentation of patients with undiagnosed TB, slow diagnosis of their TB, and failure to isolate them appropriately.[11,12] Efforts have been made to improve infection control procedures to try to reduce the risk of nosocomial spread of TB within the EC.[11,13] A search using Ovid Medline did not find any comparable work from SA.
Motivation
George Regional Hospital (GRH) is a 272-bed regional referral hospital for the Eden and Central Karoo districts of the Western Cape, covering a mixed urban and rural population of 500 000 people. The Western Cape is an area with a high prevalence of TB. The GRH EC is the only public healthcare facility in the George district that provides a 24-hour service, with approximately 3 200 attendances a month. The perception among EC staff is that a high burden of PTB is being diagnosed and infection control procedures are lacking, resulting in a high risk of nosocomial transmission. Our literature search found that while there has been research in SA looking at the risk of nosocomial transmission of TB, none has been carried out in the setting of the EC, identified internationally as a high-risk area.
Objectives
To focus on establishing the burden of PTB presenting to GRH via the EC and then auditing current IPC practice regarding patients with PTB (using the CDC audit tool[3] as a guide). This would confirm whether the EC is a high-risk environment for the transmission of PTB, as demonstrated in high-income countries, and assess the adequacy of current IPC policy to prevent transmission. If this was found to be inadequate, simple IPC measures as outlined in the WHO policy could be implemented with a view to undertaking a re-audit to determine whether IPC had improved.
Ethical approval was granted by the Stellenbosch University Health Research and Ethics Committee 2 on 3 December 2014 (protocol No. N14/10/153).
Methods and discussion
Phase 1: Baseline assessment
National data from the National Health Laboratory Service[14] showing the number of patients diagnosed with TB on Xpert MTB/RIF were used to calculate the burden of PTB in the EC and relate this to the burden in the George and Eden districts. According to these data, 20% of PTB diagnosed in the George District is at GRH; this constitutes 7% of PTB diagnosed in the Eden District (Table 1).
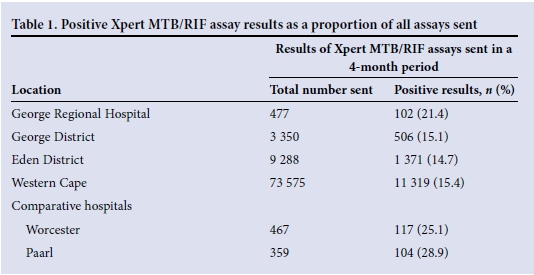
A cohort of patients was then created using the monthly facility alert organism report and International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD-10) coding from the monthly GRH Outpatient Department Appointment Clinicom data spreadsheet. This included: (i) all patients with a positive Xpert MTB/ RIF assay test for Mycobacterium tuberculosis (MTB); (ii) all patients with a positive finding on direct sputum microscopy for acid-fast bacilli; and (iii) all patients with an ICD-10 code that correlates with a diagnosis of TB (A15-19), and excluded: (i) patients who on review of clinical records were not seen in the EC; and (ii) patients who were incorrectly coded and did not have PTB.
A cohort of 102 patients was created using data from August and September 2014, of whom 81 had confirmed TB. Five of these patients had drug-resistant TB, and 40% had attended the hospital in the previous 6 months.
Data on IPC in the EC were then collected for 1 week on the twice-daily ward rounds. For each patient with suspected or confirmed PTB, the IPC measures in place were assessed. It was found that there were 32 patient encounters in 1 week: 22% of patients were in an isolation room, 66% were in an open bay but wearing a surgical mask, and 12% had no IPC measures in place. Only the patients in the isolation room had any signage identifying them as requiring IPC measures. Of these patients, 35% were positive on Xpert MTB/RIF assay, confirming the suspicion that high numbers of patients with PTB are in the EC on a daily basis with no infection control measures in place.
Based on the CDC infection control audit tool,[3] a full audit of administrative, environmental and personal protective measures in the EC was carried out. This included a review of all records of 102 adult patients with suspected TB in the EC to determine patient flow and reasons for delay.
The key issues identified were: (i) poor flow to discharge through the EC, especially after initial review by a doctor (see Table 3); (ii) delay in waiting for Xpert MTB/RIF assay results that meant patients were waiting longer in the department for a definite diagnosis; (iii) no knowledge of how the airflow systems in the EC worked or whether they worked correctly; and (iv) masks being worn incorrectly by staff of all grades.

Phase 2: Interventions Improving patient flow
The baseline data were reviewed to determine which patients stayed overnight in the EC, were admitted, or were discharged to an outpatient clinic. It was found that the standard SA triage scale[15] used in the EC was not a good predictor of admission in this group of patients. Although patients were triaged red, often with a high triage early warning score, owing to the chronic nature of their infection they were typically well compensated and not clinically acutely unwell. A set of criteria were designed that could be used by doctors when first seeing a patient to assess whether the patient needed referral or could be discharged (Fig. 1).

Once these criteria were finalised, a new standard operating procedure (SOP) for patients with suspected PTB in the EC was designed (Fig. 1). The aim was to provide criteria for patients needing admission and a protocol for their isolation and referral. If a patient did not need to be admitted, a separate protocol to facilitate a speedy discharge from the EC was drawn up. A shortage of isolation rooms in the hospital was identified, especially in the internal and family medicine wards. A system to allow cohort isolation of patients with TB was incorporated into the SOP to try to improve the flow of patients and avoid blockage of isolation rooms by long-staying patients with confirmed TB.
The delay in sputum Xpert MTB/RIF assay results was also addressed. It was decided that if a patient clearly needed admission, whether he/she had confirmed PTB based on sputum Xpert MTB/RIF or not, referral should take place and the results of Xpert MTB/RIF were not needed to determine admission. It was agreed that sputum Xpert MTB/RIF assay tests for patients who were being admitted to the hospital (those in which the results would affect decisions on isolation) could be marked as 'urgent', and the laboratory staff could be requested by phone to run the test on the next batch as a priority.
This pathway was agreed upon by consultant staff in internal, family and emergency medicine and then presented to the junior staff in all departments before implementation. The policy was approved by hospital management for use and uploaded to the Electronic Capture Medium (ECM) system. It was also made available as a physical copy in the EC for ease of reference, and in the intern handbook.
Improving communication with primary care
A need was identified by staff working in the EC, and also the staff working in the Harry Comay (TB) Hospital and clinics (which receive many of the discharged patients), to have a standard discharge summary for patients with TB that contained the key information required and was quick to fill in/read. It was also identified that there was no safety net for patients once discharged from the EC if they did not present to a clinic.
In consultation with clinical staff in the hospital and colleagues at Harry Comay Hospital, a new discharge summary template was created. This could be filled in electronically or printed, and options were given for key information that could be deleted as appropriate to decrease the time needed to fill it in. It was revised according to feedback from all staff and then made available on the ECM computer system for use.
To increase the ease of communication and provide a 'safety net', an email system was created. A generic email was made available through the Outlook Web App on all computers in the EC and in the family medicine and internal medicine wards. The email address book was populated with email addresses for each primary healthcare clinic, Harry Comay Hospital and the HAST (HIV, sexually transmitted infections, TB) co-ordinator. This email was then used to send discharge summaries in real time to the clinic where the patient was to be followed up, allowing the nursing staff to trace any patient who failed to arrive. Training was given to all staff in the EC and instructions were made available on the computers.
Improving the use of N95 masks
A teaching session was designed and delivered to the EC staff on correct use of N95 masks. A poster with key information on it was also designed and printed in the EC handbook and made available in the EC as a reminder.
Updating infection control policy
The infection control policy for TB at GRH was updated to reflect current knowledge and include the new protocols in the EC. This was approved by the quality assurance manager and accepted officially in the hospital.
Phase 3: Reassessment
Using the same method as outlined in the baseline assessment, the flow of patients through the EC was re-audited to determine whether there had been a change following implementation of the new policy. Patients seen in December 2014 and early January 2015 were selected using the same criteria as outlined in baseline assessment.
The longest length of stay of a patient was reduced by 40% (from 142 to 84 hours), and the average time between seeing a doctor and leaving the EC was 20% shorter (Tables 2 and 3). On reassessment of patient records, it was clear that there had been a reduction in the time patients with PTB spent in the EC. There was a much higher awareness and understanding among staff of the risk of TB and the preventive measures to be taken, including swift identification on ward rounds of patients needing to be moved into isolation and those for whom early discharge was appropriate.
Conclusion
The implementation of simple measures in assessment and management of patients with suspected TB in the EC significantly reduced the length of patient stay. This potentially reduces the risk of transmission of TB to both staff and patients. The data collected in the reassessment phase included the festive period and yearly staff changeover, both extremely busy times of year in the EC. If reassessed again in a few months, the length of stay may decrease even further.
Further improvements, e.g. creation of a sputum collection room and regular assessment of the ventilation system in the EC, would probably further decrease the infection risk in the EC and will be studied in the near future.
References
1. Groenewald P, Berteler M, Bradshaw D, et al. Western Cape Mortality Profile 2010. Cape Town: South African Medical Research Council, 2013. [ Links ]
2. Western Cape Government Health. Healthcare 2030: The Road to Wellness. Cape Town: Western Cape Government Health, 2014:66-68. [ Links ]
3. Centers for Disease Control and Prevention. Guidelines for Preventing the Transmission of Mycobacterium tuberculosis in Health-Care Settings, 2005. Atlanta, GA: MMWR, 2005:54(No. RR-17), Appendix B. [ Links ]
4. Stop TB Department. WHO Policy on TB Infection Control in Health-Care Facilities, Congregate Settings and Households. Geneva: World Health Organization, 2009. [ Links ]
5. Farley J, Tudor C, Mphahlele M. A national infection control evaluation of drug-resistant tuberculosis hospitals in South Africa. International Journal Tuberculosis and Lung Disease 2012;16(1):1-16. [http://di.doi.org/10.5588/ijtld.10.0791] [ Links ]
6. Claassens MM, van Schalkwyk C, du Toit E, et al. Tuberculosis in healthcare workers and infection control measures at primary healthcare facilities in South Africa. PLoS One 2013;8(10):1-8. [http://dx.doi.org/10.1371/journal.pone.0076272] [ Links ]
7. Mphahlele MT, Tudor C, van der Walt M, Farley J. An infection control audit in 10 primary health-care facilities in the Western Cape Province of South Africa. Int J Infect Control 2012;8(3):8-12. [http://dx.doi.org/10.3396/ijic.v8i3.10303] [ Links ]
8. Joshi R, Reingold AL, Menzies D, Pai M. Tuberculosis among health-care workers in low- and middle-income countries : A systematic review. PLoS Med 2006;3(12):e494. [http://dx.doi.org/10.1371/journal.pmed.0030494] [ Links ]
9. Sissolak D, Bamford CM, Mehtar S. The potential to transmit Mycobacterium tuberculosis at a South African tertiary teaching hospital. Int J Infect Dis 2010;14(5):e423-8. [http://dx.doi.org/10.1016/j.ijid.2009.06.030][PMID: 19889562] [ Links ]
10. Van Rie A, McCarthy K, Scott L. Prevalence, risk factors and risk perception of tuberculosis infection among medical students and healthcare workers in Johannesburg, South Africa. S Afr Med J 2013;103(11)853-857. [http://dx.doi.org/10.7196/samj.7092] [ Links ]
11. Behrman A, Shofer F. Tuberculosis exposure and control in an urban emergency department. Ann Emerg Med 1998;31(3):3-8. [http://dx.doi.org/10.1016/S0196-0644(98)70349-X] [ Links ]
12. Moran GJ, McCabe F, Morgan MT, Talan DA. Delayed recognition and infection control for tuberculosis patients in the emergency department. Ann Emerg Med 1995;26(9):290-295. [http://dx.doi.org/10.1016/S0196-0644(95)70074-9] [ Links ]
13. Sokolove PE, Lee BS, Krawczyk JA, et al. Implementation of an emergency department triage procedure for the detection and isolation of patients with active pulmonary tuberculosis. Ann Emerg Med 2000;35(4):327-336. [http://dx.doi.org/10.1016/S0196-0644(00)70050-3] [ Links ]
14. National Health Laboratory Service. NPP GeneXpert Summary Report 01 March 2011 to 30 June 2014. Johannesburg: NHLS, 2014. [ Links ]
15. South African Triage Group. The South African Triage Scale (SATS). 2012. http://emssa.org.za/sats/(accessed 5 January 2015). [ Links ]
Corresponding author: H Casey (helenrosecasey@gmail.com)














