Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.105 n.10 Pretoria Oct. 2015
http://dx.doi.org/10.7196/SAMJNEW.7802
FORUM
CLINICAL ALERT
Non-typhoidal Salmonella infections in HIV-positive adults
E L Subramoney
Medical officer in the Department of Internal Medicine at Mahatma Gandhi Memorial Hospital, Durban, South Africa. Her areas of special interest include infectious diseases and emergency medicine
ABSTRACT
Non-typhoidal salmonellae are important pathogens causing bacteraemia especially in immunocompromised patients, but there are limited data explicitly describing the clinical characteristics and outcome in these individuals. Recurrent invasive salmonellosis has been recognised as an AIDS-defining condition in HIV-positive patients since the 1980s. Salmonella meningitis is an infrequent complication of Salmonella sepsis, accounting for 0.8 - 6% of all cases of bacterial meningitis, and is associated with a high mortality rate.
Non-typhoidal salmonellae (NTS) are increasingly recognised as important pathogens causing bacteraemia, especially in immunocompromised patients.[1] However, there are limited data explicitly describing the clinical characteristics and outcome in this cohort of individuals.[2] Since the 1980s, recurrent invasive salmonellosis has been recognised as an AIDS-defining condition in HIV-positive patients.[3]Salmonella meningitis, a rare complication of Salmonella sepsis accounting for 0.8 - 6% of all cases of bacterial meningitis,[4] was first reported by Ghon in 1907 (cited by Ohaiseadha et al.[5]). While relatively infrequent in adults, even those who are HIV-infected, meningitis due to Salmonella species is associated with a high mortality rate.[1]
Case presentation
A 33-year-old woman had tested HIV-positive in 2011; her baseline CD4 count was unknown. She had received antiretroviral therapy (ART) since 2011 (initial regimen unknown, changed to a fixed-dose combination drug in August 2013). At that time her CD4 count was 67 cells/µL. She had had pulmonary tuberculosis in 2012 and had completed 6 months of antituberculosis treatment. At the time of her admission in July 2014, her CD4 count was 96 cells/µL and her HIV viral load was suppressed. She reported having had a generalised headache for 5 days, and had a longstanding history of visual impairment. Her vision had deteriorated acutely 2 days before admission. There was no associated vomiting, fever, photophobia, confusion or seizures. She had a recent history of constipation but no diarrhoea or abdominal discomfort.
Clinically, the patient was chronically ill with generalised wasting. Recorded vital signs on admission were essentially normal apart from sinus tachycardia. She had pallor, clubbing and significant lymphadenopathy, but no oral candidiasis.
Central nervous system assessment revealed that the patient was conscious, alert and fully orientated. She had terminal nuchal rigidity. She was blind and had no perception of light; fundal examination showed pallor of both optic discs, the left being more affected than the right. There were no gross motor or sensory deficits, but her gait was not assessed owing to her visual deficit. Findings on examination of the other systems were unremarkable.
The working diagnosis was acute meningitis, and intravenous (IV) ceftriaxone 2 g 12-hourly was empirically commenced, pending results of a lumbar puncture (LP).
Blood investigations were undertaken. An enhanced computed tomography (CT) scan of the brain showed no intracranial abnormality. As there were no contraindications, a diagnostic LP was performed. Blood cultures and stool microscopy studies were negative, as was syphilis serology. An ophthalmological assessment led to the conclusion that the blindness was due to bilateral disc atrophy secondary to meningitis.
Cerebrospinal fluid (CSF) findings on admission and after 12 days of antibiotic treatment are shown in Table 1.
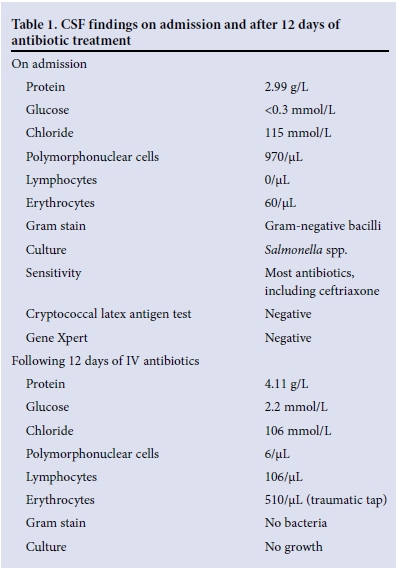
The patient completed 2 weeks of IV ceftriaxone. In view of the CSF findings following ceftriaxone therapy, she was commenced on antituberculosis therapy with steroids.
The salmonellae
Salmonellae are Gram-negative, facultative anaerobic bacilli that can colonise a wide range of mammalian hosts. Two distinct species exist, S. enterica and S. bongori. Most of the isolates that are pathogenic in humans belong to S. enterica, comprising more than 2 600 different serovars differentiated by their antigenic presentation.[6]
S. enterica are food-borne pathogens capable of causing both intestinal and extraintestinal infections in humans. The innate virulence of each serovar and host resistance are key determinants of clinical manifestations.[7] Human infections are classically divided into diseases caused by typhoidal salmonellae or NTS. The typhoidal serotypes (S. typhi and S. paratyphi) are restricted to human hosts and are highly invasive, but are rarely associated with immuno-suppression. In contrast, NTS have a more severe and invasive presentation in immunocompromised adults.[1,7]
Burden of disease and epidemiology
The burden of NTS is significant, with an estimated 93.8 million cases and 155 000 deaths globally each year.[6] Despite global morbidity, mortality is primarily restricted to the developing world. In sub-Saharan Africa, invasive NTS is a major cause of bacteraemia in adults and children,[7] with an estimated annual incidence of 175 -388/100 000 children aged 3 - 5 years and 1 800 - 9 000/100 000 HIV-infected adults.[7,8] Case fatality and relapse rates are high,[7] antibiotic resistance is an increasing problem,[9] and no vaccine is currently available.'61 Despite evidence that the initiation of ART in HIV-infected adults leads to a reduction in recurrent invasive NTS disease, there are no population data to demonstrate the impact of ART on the incidence of NTS disease.[7]
The incidence of NTS is highest during and after the rainy season in tropical climates and during the warmer months in temperate climates.[8]
Certain NTS serovars cause extraintestinal infections, the serotypes varying in different countries. The most common serotypes across Africa are S. typhimurium and S. entiritidis, although investigators at some sites report contributions from other serotypes such as S. isangi in South Africa, S. concord in Ethiopia and S. Stanleyville and S. dublin in Mali.[8] Studies conducted in Malawi and Kenya have isolated a novel variant of S. typhimurium (ST 313), which has frequently been implicated in cases of invasive NTS in the sub-Saharan region.[9]
Pathogenesis
Salmonellae are typically acquired from the environment by oral ingestion of contaminated water or food, or through contact with a carrier. Following ingestion, the bacteria bypass gastric defences, multiply, and penetrate the intestinal mucosa. They survive within the macrophages of the reticuloendothelial system and disseminate via the systemic circulation, thereby causing infection.[10] Cell-mediated immunity is vital for defence against intracellular bacteria such as salmonellae, and leucocytes, especially lymphocytes, are essential to host defence.[1] The ability of salmonellae to persist depends on a balance between immune responses that lead to the clearance of the pathogen and avoidance of damage to host tissues. [10] In their study evaluating the long-term relationship of Salmonella with its host, Ruby et al.[10] showed that the balance of T-helper cells subsets 1 and 2 (Th1/Th2) is crucial for the maintenance of bacterial persistence and survival of the host, as a strong Th1 response leads to clearance of the bacteria, while Th2 skewing leads to susceptibility and death of the host.[10]
Three essential immunological defects can probably explain the invasive pathogenesis[8] and high rate of recurrence of NTS in HIV-infected adults.[7] The first involves the gastrointestinal mucosa, which is one of the earliest and most affected sites of CD4 T-cell depletion, especially of interleukin-17-producing T cells (Th17 cells), in HIV infection.[7,8] Th17 cells and their associated family of cytokines are essential for the integrity, repair and maintenance of the epithelial mucosal barrier and enable epithelial cell expression of antimicrobial peptides,[8] thereby playing a vital role in controlling local invasion by Salmonella. Moreover, Th17 cells have an important role in the stimulation of innate immune responses, by mediating neutrophil chemoattraction and function. Secondly, cytokine responses are crucial for control of intracellular Salmonella infection. However, in advanced HIV there is dysregulated cytokine production during intracellular infection, which appears to allow persistence and recurrence of invasive NTS.[1] Thirdly, the significance of antibodies is increasingly recognised, both for serum killing and intracellular oxidative killing of invasive Salmonella. Antibodies directed against Salmonella lipopolysaccharide are shown to impair serum killing by blocking or competing with coexisting effective bactericidal antibodies directed against outer membrane proteins of Salmonella.[7]
Clinical presentation
Clinical manifestations range from subclinical infection to severe life-threatening disease, viz. enteric (68%), Salmonella sepsis (8%), non-enteric focal infections (7%, including meningitis (0.8%)), and a chronic carrier state (15%).[5]
NTS infections present primarily with a self-limiting enterocolitis, which often does not require antimicrobial therapy; invasive NTS infections manifest with high fever, hepatosplenomegaly and respiratory complications, with intestinal symptoms often being absent.'81 When Salmonella bacteria enter the systemic circulation, all tissues and organs are susceptible, leading to focal Salmonella infections, including meningitis.[4] It is postulated that high-grade bacteraemia is required for meningeal invasion, although in the case of NTS this bacteraemia may not be detected in up to 40% of cases.[3] Clinical manifestations of NTS meningitis appear to be typical for bacterial meningitis. The mortality rate associated with NTS focal infections is high, in the order of 30% in published series, particularly in the case of meningitis, when it approaches 50%.[3] Impairment of consciousness at presentation has been cited as a risk factor for mortality in such cases,[3] and of patients who do survive, 43% are left with permanent neurological deficits.[4] Ventriculitis, subdural empyema, hydrocephalus and rarely brain abscess are recognised acute neurological complications of Salmonella meningitis.[4]
Chronically infected hosts are often asymptomatic and transmit disease to naive hosts via faecal shedding of bacteria, thereby serving as a critical reservoir for disease.[10] However, despite symptoms lasting only for a few days, adults may excrete Salmonella for an average of a month following infection, while children under the age of 5 years shed bacteria in their faeces for an average of 7 weeks.[11] Several studies have shown that treatment with antibiotics can prolong shedding of NTS bacteria,[11] although these findings are controversial.[6]
Our patient had no history suggestive of a preceding bout of enterocolitis, yet she had clinical features in keeping with meningitis. It is likely that she had significant bacteraemia (on account of the organism being isolated from the CSF), but was one of the 40% of reported cases in whom this may not be detected. Stool microscopy studies were also negative, which eliminated her as a carrier.
Investigations
The diagnosis of NTS infection is based on isolation of the organism from freshly passed stool, or from blood or any other normally sterile body fluid. All salmonellae isolated in clinical laboratories should be sent to local public health departments for serotyping.[3]
Once a working diagnosis of meningitis is made, an LP is the key investigation to confirm the clinical suspicion. It is vital to bear in mind the contraindications to such a procedure, especially in resource-limited settings where there may not be ready access to a CT scan facility. According to recently published guidelines for the management of acute meningitis,[12] isolated cranial nerve palsies are not a contraindication to LP, but there is need for vigilance when the patient has a concomitant depressed level of consciousness.
The CSF is usually normal or reveals mild pleocytosis, elevated protein and a decreased glucose level.[3] The serotypes isolated from CSF are similar to those causing bacteraemia, and it is has therefore been postulated that no serotype has a particular predilection for the central nervous system.[13]
Treatment
Uncomplicated NTS gastroenteritis should be managed sympto-matically, as the symptoms are usually self-limited and the duration of fever and diarrhoea is not significantly decreased by antibiotic therapy; however, antimicrobials may be considered in patients at increased risk of invasive NTS infections.[11]
There are no studies indicating the best combined antimicrobial and ART regimen to treat acute infection and prevent relapse.[8] The Infectious Disease Society of America guidelines[8] for the management of invasive NTS in HIV-infected adults recommend 2 - 6 weeks of fluoroquinolone therapy.[8] Feasey et al.[8] propose that rapid initiation of ART may prevent relapse. Moreover, zidovudine has been documented to show anti-Salmonella activity in vitro and appears to be clinically useful in the prevention of relapse of infection.[11]
In Africa and most other developing regions, multidrug resistance is an escalating problem and is linked to poor disease outcome.[6] Currently the fluoroquinolones are the first-line agents for salmon-ellosis, given their efficacy against intracellular Gram-negative bacteria,[7] with third-generation cephalosporins reserved as second-line agents. Azithromycin (a macrolide) has shown activity against both nalidixic acid-resistant and multidrug-resistant strains.[6]
Treatment of Salmonella meningitis is very difficult, and has never been standardised; however, it is recommended that antimicrobial therapy be used for 4 weeks.[13] Prolonged therapy for Salmonella meningitis is indicated in view of slow response and the high probability of relapse (~64%).[4] Various alternative drugs have been used during recent decades, including chloramphenicol, ampicillin, co-trimoxazole, third-generation cephalosporins and fluoroquinolones, with the former three producing unsatisfactory results.[13] For meningitis or deep CNS involvement, high-dose ceftriaxone is the best choice for optimal penetration of the blood-brain barrier.[11] There are currently no data to suggest that combination therapy (e.g. a fluoroquinolone with a third-generation cephalosporin) is more effective than either as a single agent.[11,13]
Our patient's treatment regimen unfortunately spanned a period of only 2 weeks, as the finding of sterile CSF following only 12 days of IV ceftriaxone led us to be satisfied with progress and therefore to conclude prematurely that she had been adequately treated. According to suggested guidelines, therapy should have been prolonged for a further 2 weeks. Of note, she had been on ART since 2011 and her HIV viral load was supressed, yet she still acquired an opportunistic NTS infection reflecting her immunosupression (and low CD4 count of only 96 cellsµL).
Our decision to commence antituberculosis therapy was prompted by the last LP findings (high CSF protein, low CSF glucose, and a predominant lymphocytosis), her immunocompromised state and chronic tuberculous meningitis being the most likely explanation for her loss of vision.
Disclaimer. The views expressed in this article are the author's own and not an official position of Mahatma Gandhi Memorial Hospital.
Acknowledgment. The author thanks Dr Susan Brown for proofreading the article.
References
1. Gordon MA. Salmonella infections in immunocompromised adults. J Infect 2008;56(6):413-422. [http://dx.doi.org/10.1016/j.jinf.2008.03.012] [ Links ]
2. Dhanoa A, Fatt QK. Non-typhoidal Salmonella bacteremia: Epidemiology, clinical characteristics and its association with severe immunosuppression. Ann Clin Microbiol Antimicrob 2009;8:15. [http://dx.doi.org/10.1186/1476-0711-8-15] [ Links ]
3. Belloso WH, Romano M, Greco GS, et al. Recurrent meningitis and subarachnoid haemorrhage due to Salmonella in an HIV+ patient: Case report and mini-review of the literature. Open AIDS J 2011;5:62-66. [http://dx.doi.org/10.1186/1476-0711-8-1510.2174/1874613601105010062] [ Links ]
4. Fuad Khalil AA, Salem A, Rafid A, et al. Salmonella meningitis in an adult with type B viral hepatitis and an incidental schwannoma. BMJ Case Rep 2009;2009:bcr11.2008.1209. [http://dx.doi.org/10.1136/bcr.11.2008.1209] [ Links ]
5. Ohaiseadha CO, Dunne OM, Desmond F, et al. Salmonella meningitis and septicaemia in an non-immunocompromised adult, associated with a cluster of Salmonella enteriitidis PT 14b, Ireland, November 2009. Euro Surveill 2010;15(7):pii=19489. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19489 (accessed 7 September 2015). [ Links ]
6. Gal-Mor O, Boyle EC, Grassi GA. Same species; different diseases: How and why typhoidal and non typhoidal Salmonella enterica serovars differ. Front Microbiol 2014;4(5):391. [http://dx.doi.org/10.3389/fmicb.2014.00391] [ Links ]
7. Gordon MA. Invasive non-typhoidal Salmonella disease - epidemiology, pathogenesis and diagnosis. Curr Opin Infect Dis 201134(5):484-489. [http://dx.doi.org/10.1097/QCO.0b013e32834a9980] [ Links ]
8. Feasey NA, Dougan G, Kingsley RA, et al. Invasive non-typhoidal Salmonella disease: An emerging and neglected tropical disease in Africa. Lancet 2012;379(9835):2489-2499. [http://dx.doi.org/10.1016/S0140-6736(11)61752-2] [ Links ]
9. De Jong HK, Parry CM, van der Poll T, et al. Host-pathogen interaction in invasive salmonellosis. PLoS Pathog 2012;8(10):e1002933. [http://dx.doi.org/10.1371/journal.ppat.1002933] [ Links ]
10. Ruby T, McLaughlin L, Gopinath S, et al. Salmonella's long-term relationship with its host. FEMS Microbiol Rev 2012;36(3):600-615. [http://dx.doi.org/10.1111/j.1574-6976.2012.00332.x] [ Links ]
11. Hohmann EL. Non typhoidal salmonellosis. Clin Infect Dis 2001;32(2):263-269. [http://dx.doi.org/10.1086/318457] [ Links ]
12. Boyles TH, Bamford CM, Bateman K. Guidelines for the management of acute meningitis in children and adults in South Africa. South Afr J Epidemiol Infect 2013;28(1):5-15. [ Links ]
13. Owusu-Ofori A, Scheld WM. Treatment of Salmonella meningitis: Two case reports and a review of the literature. Int J Infect Dis 2003;7(1):53-60. [http://dx.doi.org/10.1016/S1201-9712(03)90043-9] [ Links ]
Accepted 25 August 2015.
Corresponding author: E L Subramoney (evette.subramoney238@gmail.com)














