Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SAMJ: South African Medical Journal
versión On-line ISSN 2078-5135
versión impresa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.105 no.9 Pretoria sep. 2015
http://dx.doi.org/10.7196/SAMJNEW.8426
CONTINUING MEDICAL EDUCATION
ARTICLE
Pharmacological management of chronic obstructive pulmonary disease
E ShaddockI; G RichardsII
IMB BCh, FCP (SA), Cert Pulmonology (SA), Cert Critical Care (SA). Division of Pulmonology and Critical Care, Department of Medicine, Faculty of Health Sciences, University of the Witwatersrand and Charlotte Maxeke Johannesburg Academic Hospital, Johannesburg, South Africa
IIMB BCh, PhD, FCP (SA), FCCP, FRCP. Critical Care, Faculty of Health Sciences, University of the Witwatersrand and Charlotte Maxeke Johannesburg Academic Hospital, Johannesburg, South Africa
ABSTRACT
There have been significant changes in the approach to the management of chronic obstructive pulmonary disease (COPD) over the past decade. The World Health Organization suggests four components to a COPD management plan: (i) assess and monitor disease; (ii) reduce risk factors; (iii) manage stable COPD; and (iv) manage exacerbations. Encouraging patients to limit their risk exposure is essential, whether it be smoking cessation or removing exposure to biomass.
The main objective of treatment is to relieve daily symptoms, improve quality of life and importantly decrease the risk of future exacerbations. Current guidelines are based on grade A and B evidence. Pneumococcal and annual influenza vaccinations are encouraged. A holistic approach that augments pharmacological treatment includes good nutrition and pulmonary rehabilitation.
Bronchodilators are the cornerstone of management. Depending on the patient's placement in the GOLD ABCD classification, treatment is individualised. Short-acting bronchodilators are used as rescue medication, while long-acting bronchodilators or/and long-acting muscarinic agents are the treatment of choice for patients in groups B, C and D. Inhaled corticosteroids are only recommended for groups C and D. Most patients respond well to combinations of the abovementioned medications. For patients who still have frequent exacerbations, alternative choices include long-term macrolides andphosphodiesterase 4 inhibitors.
Over the past decade there have been significant changes in the approach to the management of chronic obstructive pulmonary disease (COPD). Previously, drugs were used that had originally been developed for asthma and were relatively nihilistic with regard to COPD management. Currently, the availability and use of drugs that have been developed primarily for the COPD patient have become mainstream treatment. There is now more optimism and empathy towards this rapidly growing group of patients.
According to 2004 World Health Organization (WHO) estimates, 64 million people have COPD and 3 million have died of the condition. The WHO also predicts that COPD will become the third leading cause of death worldwide by 2030.[1] It suggests that there are four very useful components to a COPD management plan: (i) assess and monitor disease; (ii) reduce risk factors; (iii) manage stable COPD; and (iv) manage exacerbations.
Assess and monitor disease
The correct diagnosis of COPD and its severity is essential if the appropriate treatment is to be prescribed. If COPD is suspected after taking a history and performing a clinical examination, it should be confirmed with spirometry. A forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) of <0.7, with no or minimal reversibility after the administration of an inhaled short-acting bronchodilator, is in keeping with the diagnosis. The new GOLD (Global Initiative for Chronic Obstructive Lung Disease) guidelines'21 have added symptoms and risk for exacerbations into the classification, which allows for a more evidence-based approach when deciding on appropriate medications.
Reduce risk factors
It is essential to discuss modifying or removing risk factors for COPD in all patients who have been diagnosed with the condition, as ongoing exposure to a risk factor results in a more rapid decline of FEV1. This most frequently involves advice on smoking cessation or attempts to reduce biomass exposure. Smoking cessation programmes have significant benefit to patients who have decided to quit the habit.
Manage stable COPD
Bronchodilators
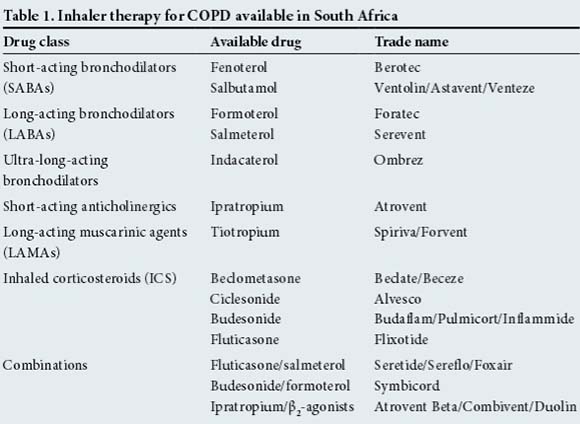
Except in patients with very mild disease and minimal symptoms, short-acting bronchodilators (SABAs) alone are no longer routinely recommended for COPD. SABAs and short-acting anticholinergic agents are used 'as required rescue medications' and not for maintenance therapy. The backbone of treatment for most COPD patients is long-acting β2-agonists (LABAs) and long-acting muscarinic antagonists (LAMAs). These agents have been shown to provide improvements in dyspnoea, health-related quality of life (QoL), lung function, rescue medication use, exercise capacity and exacerbation risk.[3-7] The decision as to which agent to start with is often based on patient preference, available finances (LABAs are usually less expensive than LAMAs) and preferred delivery device. There are many drug choices and brands to choose from (Table 1).
Over the past 10 years evidence has been emerging with regard to bronchodilator maintenance therapy in COPD patients. Large randomised studies such as TORCH and UPLIFT, with long-term follow-up of 3 and 4 years, respectively, have become landmark studies and the evidence on which many guidelines are based.[8,9] Bronchodilators are effective in most patients with COPD and not only in those with reversible disease on spirometry. An important benefit of LABAs and LAMAs is their effect on decreasing dynamic hyperinflation, which directly affects QoL.
The TORCH study compared four groups, placebo v. salmeterol and fluticasone alone and the latter v. a salmeterol-fluticasone combination (SFC) over 3 years (6 112 patients).[8] The primary outcome was death from any cause for the comparison between the combination regimen and placebo. The study also assessed frequency of exacerbations, health status and lung function over the 3 years. The results showed that the all-cause mortality was 12.6% in the combination therapy group, 15.2% in the placebo group, 13.5% in the salmeterol group, and 16.0% in the fluticasone group. The hazard ratio for death in the combination therapy group compared with the placebo group was 0.825 (95% confidence interval (CI) 0.681 - 1.002; p=0.052), corresponding to a 17.5% reduction in the risk of death.[8] This did, however, not meet the predetermined level of statistical significance (p=0.052). Even though not a primary outcome, it is important to note that SFC, compared with placebo, decreased the annual rate of exacerbations from 1.13 to 0.85 and improved health status and lung function (p<0.001 for all comparisons with placebo).[8] There was, however, an increase in the risk of pneumonia in the groups that received inhaled corticosteroids (ICS); this will be discussed in more detail below.
UPLIFT was a 4-year randomised, doubleblind trial, which compared either tiotropium (a LAMA) or placebo in patients with COPD.[9] Importantly, 75% of patients in both groups were already on LABAs and ICS; therefore, any benefit was over and above that of standard therapy. The primary endpoints were the rate of decline in the mean FEV1 before and after bronchodilator use, starting on day 30. Secondary endpoints included measures of FVC, changes in St George's Respiratory Questionnaire (SGRQ) score, number and timing of acute exacerbations of COPD and mortality. The study did not find a significant difference between the groups in the rate of decline of FEV1. However, there was a significant improvement in the SGRQ in the tiotropium group compared with the placebo group at each time point throughout the 4 years (ranging from 2.3 U to 3.3 U; p<0.001).[9] At 4 years, tiotropium was also associated with a reduction in the risk of exacerbations, related hospitalisations, and respiratory failure.[9] There was no benefit in its primary outcome; however, all its secondary outcomes showed improvement. If we consider the main complaints of COPD patients, this drug was shown to have a real-life benefit.
LABAs can be used safely without ICS in COPD compared with asthma. When adding an ICS in COPD, it is necessary to decide if the benefit will be greater than the increased risk of pneumonia. ICS in combination with LABAs has been shown in some studies to improve lung function and QoL and reduce exacerbations.[8,10] The best evidence for these benefits, however, is in patients with >2 exacerbations per year, especially in GOLD groups C and D. In patients without frequent exacerbations, these agents should not be initiated or should be gradually discontinued owing to the risk of pneumonia. There have been concerns about withdrawal of ICS in patients who have received the medication inappropriately or who are stable on LABA-LAMA-ICS. However, the WISDOM study found that in patients with severe COPD, who were receiving tiotropium plus sal-meterol, the risk of moderate or severe exacerbations was similar among those who gradually discontinued ICS and those who continued with the medication. The study did, however, note a slightly greater decrease in lung function during the final step of ICS withdrawal, but it is nevertheless considered safe to discontinue these agents if their use is not indicated.[11]
The review article in this edition of CME discusses the diagnosis and classification of the severity of COPD.[12]
The majority of COPD patients, especially as the severity of the condition increases, are not usually managed with one agent only. Combination therapy, including 2 or even 3 agents, possibly in the same dispensing device, has become the ultimate management choice. It allows for improved patient compliance and disease control. There is growing interest in LABA/LAMA combinations, and as these combinations are not yet available in a single dispenser in South Africa (SA), the agents can be used together. There are numerous studies (although no large randomised controlled studies) demonstrating that combinations improve lung function and decrease exacerbation rates more than either component alone.
There is a trend in lower-income countries for ICS to be used early in the management of COPD. This is certainly true in SA, primarily because of the cost and availability in clinics and hospitals. COPD patients are mostly managed as asthmatics with ICS and SABAs, as required. This is not in the best interests of the patient; LABAs and possibly LAMAs should be available at clinic level.
Oral medications
Theophylline is still widely used as an oral bronchodilator, mainly because of its low cost and easy accessibility. Theophylline has been shown to improve QoL, but its toxicity profile limits its acceptability as a first-line agent. The SA guidelines[13] recommend considering low-dose theophylline (as an anti-inflammatory agent and with measurement of blood levels) as a treatment option, and the GOLD guidelines[2] suggest it as an alternative if no other bronchodilators are available.
Phosphodiesterase inhibitors, such as roflumilast, inhibit the airway inflammatory processes associated with COPD. They have been shown to be effective in Phase III clinical trials and have recently been registered for use in SA. In a pooled analysis of 2 large trials, a significant 17% reduction in the frequency of moderate or severe acute exacerbations (AEs) was demonstrated.[14] This drug is expensive, but has been shown to be cost-effective if used in suitable patients, although there are significant gastrointestinal side-effects.[15]
Long-term macrolide antibiotics are not currently recommended by the GOLD guidelines. However, they may be considered in patients with >2 exacerbations per year. The use of macrolides for the prevention of AEs is based on their immunomodulatory and anti-inflammatory effects, which have been long recognised in patients with cystic fibrosis and diffuse panbronchiolitis. Macrolides decrease sputum production, inhibit biofilm formation and reduce production of different virulence factors; recently, antiviral effects have also been reported.[16] There have been a number of small studies showing the benefits of long-term macrolides. A recent randomised controlled trial of 1 142 patients with a 1-year follow-up period, using azithromycin 250 mg daily, showed that the median time to first AE was 266 days (95% CI 227 - 313) in the azithromycin group and 174 days (95% CI 143 - 215) in the placebo group (p<0.001). The hazard ratio for having an AE per patient-year in the azithromycin group was 0.73 (95% CI 0.63 - 0.84; p<0.001). They also demonstrated improved QoL, but there was a small increase in the risk for hearing loss in the treatment arm.[17] The side-effects of macrolides need to be considered and monitored when initiating therapy, as the most serious side-effects include ototoxicity (hearing loss, tinnitus and vertigo), cardiac arrhythmias (especially prolonged QTc interval) and hepatotoxicity.
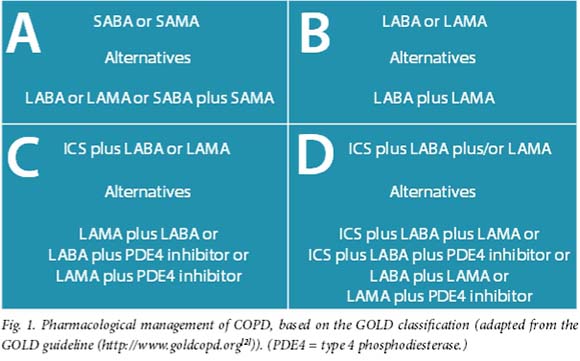
When deciding on which agent to use, the GOLD method of classifying patients into their respective ABCD group allows one to make a better evidence-based choice of agents (Fig. 1).
Inhalation device
An often forgotten component of COPD treatment is the decision about the type of inhaler device. Inhaler technique, regardless of the device, should be checked regularly at every consultation and, if incorrect, re-taught repeatedly. It is important to match the patient's ability with the correct device. Because of the lower cost, pressurised metered-dose inhalers (pMDIs) are most commonly prescribed, and if used correctly are good options. They are, however, most often incorrectly used. Dry powder inhalers (DPIs) are the inhalers of choice in patients with poor hand-lung co-ordination. DPIs are usually more expensive than pMDIs, but are very popular as patients find them easier to use. An equally important determinant of choosing a device is the patient's ability to generate adequate inspiratory flow. In advanced COPD and in those having an exacerbation, the patient may not be able to generate sufficient inspiratory pressure to trigger the release of medication in DPIs. Similarly, pMDIs may not be inhaled sufficiently deeply and nebulisation may be necessary. In advanced COPD, inspiratory muscles can become weaker and be a mechanical disadvantage in the presence of severe hyperinflation. During follow-up, inhaler choice should be regularly evaluated and a change made if necessary.
Manage exacerbations
A vital part of COPD management is to try to decrease the risk of AEs. It has been shown that AEs have a negative impact on patient prognosis. Soler-Cataluna et al.[18] showed that patients with the greatest risk of mortality were those with >3 AEs (hazard ratio 4.13; 95% CI 1.80 - 9.41). AEs also result in an accelerated decline in lung function, poor QoL and increased health resource use. As discussed above, many of the current treatments, including ICS, LABAs and LAMAs, have been shown to decrease AEs.
References
1. Burden of COPD. www.who.int/respiratory/copd/burden/en (accessed 4 July 2015). [ Links ]
2. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for Diagnosis, Management and Prevention of COPD. 2015. http://www.goldcopd.org (accessed 3 July 2015). [ Links ]
3. Keating GM. Tiotropium bromide inhalation powder: A review of its use in the management of chronic obstructive pulmonary disease. Drugs 2012;72(2):273-300. [http://dx.doi.org/10.2165/11208620-000000000-00000] [ Links ]
4. O'Donnell DE, Flüge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J 2004;23(6):832-840. [ Links ]
5. Steiropoulos P, Tzouvelekis A, Bouros D. Formoterol in the management of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2008;3(2):205-215. [ Links ]
6. Stockley RA, Whitehead PJ, Williams MK. Improved outcomes in patients with chronic obstructive pulmonary disease treated with salmeterol compared with placebo/usual therapy: Results of a meta-analysis. Respir Res 2006;7:147. [ Links ]
7. Bateman ED, Mahler DA, Vogelmeier CF, et al. Recent advances in COPD disease management with fixed-dose long-acting combination therapies. Expert Rev Respir Med 2014;8(3):357-379. [http://dx.doi.org/10.1586/17476348.2014.910457] [ Links ]
8. Calverley PMA, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007;356:775-789. [ Links ]
9. Tashkin DP, Celli B, Stephen SS, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008;359:1543-1554. [ Links ]
10. Anzueto A, Ferguson GT, Feldman G, et al. Effect of fluticasone propionate/salmeterol (250/50) on COPD exacerbations and impact on patient outcomes. COPD 2009;6:320-329. [ Links ]
11. Magnussen H, Disse B, Rodriguez-Roisin R, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med 2014;371:1285-94. [http://dx.doi.org/10.1056/NEJMoa1407154] [ Links ]
12. Viviers PJ, van Zyl-Smit RN. COPD - diagnosis and classification of severity. S Afr Med J 2015;105(9):786-788. [http://dx.doi.org/10.7196/SAMJnew.8421] [ Links ]
13. Abdool-Gaffar MS, Ambaram A, Ainslie GM, et al. Guideline for the management of chronic obstructive pulmonary disease - 2011 update. S Afr Med J 2011;101:61-73. [ Links ]
14. Calverley PM, Rabe KF, Goehring UM, et al. Roflumilast in symptomatic chronic obstructive pulmonary disease: Two randomised clinical trials. Lancet 2009;374(9691):685-694. [http://dx.doi.org/10.1016/S0140-6736(09)61255-1] [ Links ]
15. Samyshkin Y, Kotchie RW, Mörk AC, et al. Cost-effectiveness of roflumilast as an add-on treatment to long-acting bronchodilators in the treatment of COPD associated with chronic bronchitis in the United Kingdom. Eur J Health Econ 2014;15(1):69-82. [http://dx.doi.org/10.1007/s10198-013-0456-5] [ Links ]
16. Yamaya M, Azuma A, Takizawa H, et al. Macrolide effects on the prevention of COPD exacerbations. Eur Respir J 2012;40(2):485-494. [http://dx.doi.org/10.1183/09031936.00208011] [ Links ]
17. Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med 2011;365:689-698. [http://dx.doi.org/10.1056/NEJMoa1104623] [ Links ]
18. Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005;60:925-931. [ Links ]
Corresponding author: E Shaddock (eshaddock@metroweb.co.za)














