Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.105 no.9 Pretoria Set. 2015
http://dx.doi.org/10.7196/SAMJNEW.8489
CONTINUING MEDICAL EDUCATION
ARTICLE
Non-pharmacological management of chronic obstructive pulmonary disease
S AbrahamI; G SymonsII
IMB ChB, FCP (SA). Division of Pulmonology, Department of Medicine, Faculty of Medicine and Health Sciences, Stellenbosch University and Tygerberg Academic Hospital, Cape Town, South Africa
IIMB ChB, Dip PEC, FCP (SA), Cert Pulmonology (SA).Division of Pulmonology, Department of Medicine, Faculty of Health Sciences, University of Cape Town and Groote Schuur Hospital, Cape Town, South Africa
ABSTRACT
Chronic obstructive pulmonary disease (COPD) is the third leading cause of morbidity and mortality globally, contributing to a substantial use of resources. According to World Health Organization estimates, 65 million people have moderate to severe COPD. The condition is also recognised as a systemic disease with extrapulmonary manifestations, such as peripheral muscle dysfunction, malnutrition and depression, which further contribute to disability, poor quality of life, exacerbations and mortality. Optimum management requires non-pharmacological interventions combined with pharmacological treatment. However, the former is often neglected and not widely used in daily practice, with the focus mainly on the latter.
Chronic obstructive pulmonary disease (COPD) is the third leading cause of morbidity and mortality globally, contributing to a substantial use of resources.[1] According to World Health Organization estimates, 65 million people have moderate to severe COPD.[2] The condition is also recognised as a systemic disease with extrapulmonary manifestations, such as peripheral muscle dysfunction, malnutrition and depression, which further contribute to disability, poor quality of life, exacerbations and mortality. Optimum management requires non-pharmacological interventions combined with pharmacological treatment. However, the former is often neglected and not widely used in daily practice, with the focus mainly on the latter.
Non-pharmacological management options for COPD include smoking cessation, pulmonary rehabilitation, pneumococcus and influenza vaccinations, non-invasive positive pressure ventilation (NPPV), long-term oxygen therapy (LTOT), surgery and broncho-scopic lung volume reduction.
Smoking cessation remains the only proven intervention to slow the decline of lung function and should be prioritised in the management of COPD patients. A gradual decline in forced expiratory volume in 1 second (FEV1) is a normal part of ageing; however, decline is accelerated in patients with COPD, with greater decline in the early stages of COPD (Global Initiative for Chronic Obstructive Lung Disease (GOLD I and II)),[3] emphasising the importance of early smoking cessation interventions.
Pulmonary rehabilitation has been shown to be the most effective non-pharmacological intervention for improving health status and quality of life in COPD patients and addresses aspects of the disease not sufficiently covered by medical therapy, including peripheral muscle dysfunction, cachexia, social isolation and psychological issues.
Pneumoccocal and influenza vaccines may reduce infectious exacerbations - the most common cause of acute decompensation. NPPV is beneficial during acute exacerbations, reducing the need for intubation, length of stay in the intensive care unit, and mortality rate during hospitalisation. Long-term supplemental oxygen improves the mortality rate and quality of life in patients with severe resting hypoxaemia. Surgical options in COPD include lung volume reduction surgery (LVRS), bullectomy and lung transplantation. Bronchoscopic lung volume reduction is still being studied and is an emerging therapy in the management of COPD, not yet approved for use in the clinical setting. This article elaborates on the non-pharmacological interventions available for the management of COPD, which complement appropriate pharmacological therapy.
Smoking cessation
As smoking is the most important modifiable risk factor for COPD, quitting smoking is critical to reduce the occurrence and progression of the disease. Findings from landmark studies such as the Lung Health Study from the USA[4] show that long-term smoking cessation is associated with a reduction in the rate of FEV1 decline and survival. Smoking cessation not only benefits the lung (reduction in lung cancer, reduced lung function loss), but also reduces the risk of cardiovascular disease and other smoking-related conditions.
Effective smoking cessation requires a combination of pharmacological therapy and behavioural support and counselling. The South African Thoracic Society clinical practice guideline is a useful resource to assist clinicians.[5]
Pharmacological options include nicotine replacement therapies, such as nicotine gum, patches, oral inhalers and sprays, varenicline (Champix) and bupropion (Zyban). All healthcare professionals should give smoking cessation advice to every smoker at every point of contact. The 5 major steps of a smoking intervention may be summarised as 5 As:
• Ask: enquire about tobacco use during every patient contact (both current and past patterns of smoking).
• Alert: urge all smokers to quit and provide information on the benefits of quitting.
• Assess: determine willingness to make an attempt to quit.
• Assist: help the patient to quit by referral for further counselling and pharmacotherapy.
• Arrange: schedule a follow-up visit.
Pulmonary rehabilitation
Advanced COPD is associated with systemic inflammation, and increased circulating levels of cytokines and oxidative stress promote muscle loss, peripheral muscle dysfunction and cachexia. Deconditioning is associated with a shift from type I to type II fibres, which are less-endurance fibres. Furthermore, progressive dyspnoea associated with moderate to severe disease leads to limitation of physical activity, resulting in disuse atrophy and a vicious cycle of progressive deconditioning. Other factors that possibly play a role in peripheral muscle dysfunction include corticosteroid use, hypoxia and malnutrition.[6]
Pulmonary rehabilitation (PR) is a broad therapeutic concept defined as a multidisciplinary intervention comprising exercise training, education and psychological support aimed at improving quality of life and reducing disability in patients with chronic respiratory disease.[6] PR addresses extrapulmonary problems in COPD not covered by pharmacological therapies, such as exercise deconditioning, social isolation, anxiety, depression, muscle wasting and weight loss.
A comprehensive PR programme has various components and may include patient assessment, exercise training, smoking cessation, nutrition, education and psychosocial support. Multidisciplinary teams are involved, including healthcare professionals such as physicians, nurses, physiotherapists, psychologists, dieticians and social workers. Current guidelines suggest a minimum length of an effective rehabilitation programme (6 weeks), with longer programmes showing better results.[7] The optimal duration has not yet been determined. In the absence of formally structured PR programmes, home-based exercise programmes may be encouraged, e.g. leisure walking is associated with increased endurance.[8]
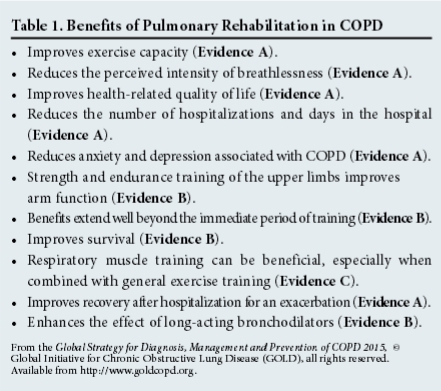
The benefits of PR are summarised in Table 1. Indications include patients with chronic respiratory impairment who, despite optimal medical management, have dyspnoea, reduced exercise tolerance or restriction of activities.[7] PR is also effective in reducing rates of hospitalisation and mortality after an acute exacerbation.[9]
Exercise training
Exercise training involves aerobic lower-extremity endurance training. Baseline exercise tolerance can be assessed by formal cardiopulmonary testing using treadmill or ergometry bicycling. Simpler methods such as the 6-minute walk test may also be used. Guidelines suggest a training frequency varying from daily to weekly, with duration ranging from 10 to 45 minutes and intensity from 50% of peak oxygen consumption to the maximum tolerated.[7,8] Interval training is another option for patients unable to tolerate continuous endurance training and consists of high-intensity exercise alternating with periods of rest or lower-intensity exercise. Resistance training provides a greater increase in muscle mass and strength than endurance training and causes less dyspnoea, making it an option for patients with severe dyspnoea and those who are hospitalised. Endurance and strength training also complement each other.[7]
Transcutaneous neuromuscular electrical stimulation (NMES) of leg muscles is a technique involving involuntary muscle contraction induced by electrical stimulation with training of selected muscle groups.[10] NMES improves leg muscle strength and reduces dyspnoea in stable patients with severe COPD and poor baseline exercise tolerance.[10] It also has added benefits and can be continued during COPD exacerbations.[11]
Some programmes include upper-extremity training, as everyday activities require the hands. However, studies have shown no benefit for dyspnoea and quality of life. Another non-conventional form of exercise includes ventilatory muscle training (studies done mostly in inspiratory muscle training), which has shown mixed results in clinical trials.
Nutritional support
Malnutrition and cachexia are common in COPD patients. Low body mass index (BMI) and fat free mass index (FFMI) are predictors of poor prognosis and associated with a higher mortality (BMI forms part of the body mass index, airflow obstruction, dyspnoea and exercise capacity (BODE) index for COPD mortality prediction). Nutritional supplementation should be considered in individuals with a BMI <21. No beneficial effects have been seen in trials investigating the use of testosterone, appetite stimulants and creatine in COPD patients with a low BMI.
Education
Patient education is an important part of PR and includes information about COPD, smoking cessation, appropriate use and administration of pharmacological therapies, decision-making during exacerbations, and advance directives, recognising and treating complications and end-of-life issues. The benefits include better adherence to treatment and fewer hospital and emergency visits.
Influenza and pneumococcal vaccinations
Acute exacerbations are a major cause of morbidity and mortality worldwide. Most acute exacerbations are triggered by community-acquired respiratory infections (viral or bacterial). Although clinical trial data are limited and not conclusive, vaccinations can prevent some of the infections that cause exacerbations. The current GOLD guidelines[3] recommend that all patients with COPD receive influenza and pnemoccocal (pneumococcal polysaccharide vaccine) vaccines, depending on availability. Small cohort studies have suggested additive benefits of administering both influenza and pneumoccocal vaccines in reducing hospitalisation and mortality.[12,13]
Non-invasive positive pressure ventilation
NPPV improves the outcome in severe exacerbations of COPD complicated by hypercapnia and respiratory acidosis. Evidence regarding long-term NPPV in COPD patients with hypercapnia is contradictory and there are no recommendations on its use.[7] An exception is the subsets of patients with COPD and coexisting obstructive sleep apnoea or obesity hypoventilation, where clear benefits are seen.
Long-term oxygen therapy
The Nocturnal Oxygen Therapy Trial (NOTT) and Medical Research Council (MRC) trials demonstrated that LTOT (>15 hours/day) is associated with improved survival in patients with severe resting hypoxaemia with no benefits seen in moderate hypoxaemia PO2 >8 kPa (60 mmHg). Indications for LTOT include the following:[7]
• PaO2 = 7.3 kPa or SaO2<88%, with or without hypercapnia.
• PaO2 between 7.3 kPa (55 mmHg) and 8 kPa (60 mmHg or SaO2 88%), if there is evidence of pulmonary arterial hypertension, peripheral oedema suggestive of congestive heart failure or polycythaemia.
Surgery
Surgical options for COPD include LVRS, bullectomy and lung transplantation.
Lung volume reduction surgery
LVRS involves the surgical reduction of lung volume, with multiple excisions improving elastic recoil and reducing hyperinflation. It is also thought to improve the mechanical function of the diaphragm and intercostal muscles by returning the diaphragm to a more normally curved and lengthened configuration. It is beneficial in selected COPD patients with upper-lobe emphysema. The National Emphysema Treatment Trial (NETT)[14] demonstrated survival benefits in patients with upper-lobe emphysema and low exercise capacity, with no benefits and increased mortality seen in FEV1 <20% predicted, diffusing capacity of the lungs for carbon monoxide <20 and non-upper-lobe emphysema on computed tomography scanning. It is, however, associated with significant morbidity postoperatively.
Bullectomy
Bullectomy involves removal of one or more large bullae that do not contribute to gas exchange, decompressing the adjacent lung parenchyma and improving ventilation. Indications include dyspnoea from giant bullae occupying >30% of the hemithorax (especially >50%), spontaneous pneumothorax, haemoptysis and repeated infections.
Lung transplantation
Lung transplantation is done infrequently in resource-limited countries such as South Africa (7 lung transplantations done in 2013). COPD is the most common indication for a single lung transplant. Indications for placing a patient with COPD on the transplant list would include a BODE index >7, FEV1 <15 - 20% of predicted, >3 exacerbations in the last year, 1 severe exacerbation with hypercapnic respiratory failure and moderate to severe pulmonary hypertension.[15]
Conclusion
Non-pharmacological interventions are complementary to pharmacological therapies in COPD. Smoking cessation is the only proven method to prevent decline in lung function and therefore must be addressed during all patient visits, with the implementation of an appropriate individually tailored pulmonary rehabilitation programme. Yearly influenza vaccination will reduce the risk of influenza with subsequent COPD exacerbations or pneumonia. Pneumococcal vaccination shows great promise and should be used where available. Keeping one's patients healthy and strong by means of nutrition and moderate exercise will contribute to their sense of wellbeing and improve their respiratory health.
References
1. Mannino DM, Buist S. Global burden of COPD: Risk factors, prevalence, and future trends. Lancet 2007;370:765-773. [ Links ]
2. World Health Organization. Chronic respiratory diseases. Burden of COPD. http://www.who.int/respiratory/copd/burden/en/ (accessed 3 August 2015). [ Links ]
3. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for Diagnosis, Management and Prevention of COPD. 2015. http://www.goldcopd.org (accessed 3 August 2015). [ Links ]
4. Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. Lung Health Study. JAMA 1994;272:1497-1505. [ Links ]
5. Van Zyl-Smit RN, Allwood B, Stickells D, et al. South African tobacco smoking cessation clinical practice guideline. S Afr Med J 2013;103:869-876. [ Links ]
6. Patel AR, Hurst JR. Extrapulmonary comorbidities in chronic obstructive pulmonary disease: State of the art. Expert Rev Respir Med 2011;5(5):647-662. [http://dx.doi.org/10.1586/ers.11.62] [ Links ]
7. Nici L, Donner C, Wouters E, et al.; ATS/ERS Pulmonary Rehabilitation Writing Committee. American Thoracic Society/ European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med 2006;173:1390-1414. [ Links ]
8. Leung RW, Alison JA, McKeough ZJ, Peters MJ. Ground walk training improves functional exercise capacity more than cycle training in people with chronic obstructive pulmonary disease (COPD): A randomised trial. J Physiother 2010;56:105-112. [ Links ]
9. Puhan M, Scharplatz MA, Troosters T, et al. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2009(3):CD005305. [ Links ]
10. Sillen MJ, Speksnijder CM, Eterman RM, et al. Effects of neuromuscular electrical stimulation of muscles of ambulation in patients with chronic heart failure or COPD: A systematic review of the English-language literature. Chest 2009;136:44-61. [http://dx.doi.org/10.1378/chest.08-2481] [ Links ]
11. Clini EM, Ambrosino N. Nonpharmacological treatment and relief of symptoms in COPD. Eur Respir J 2008;32:218-228. [http://dx.doi.org/10.1183/09031936.00134007] [ Links ]
12. Nichol KL. The additive benefits of influenza and pneumococcal vaccinations during influenza seasons among elderly persons with chronic lung disease. Vaccine 1999;17:S91-S93. [ Links ]
13. Sumitani M, Tochino Y, Kamimori T, et al. Additive inoculation of influenza vaccine and 23-valent pneumococcal polysaccharide vaccine to prevent lower respiratory tract infections in chronic respiratory disease patients. Intern Med 2008;47:1189-1197. [ Links ]
14. Weinmann GG, Chiang Y-P, Sheingold S. The National Emphysema Treatment Trial (NETT): A study in agency collaboration. Proc Am Thoracic Soc 2008;5(4):381-384. [http://dx.doi.org/10.1513/pats.200709-154ET] [ Links ]
15. Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014 - an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2015;34(1):1-15. [http://dx.doi.org/10.1016/j.healun.2014.06.014] [ Links ]
Corresponding author: S Abraham (shinu_29@yahoo.com)














