Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SAMJ: South African Medical Journal
versión On-line ISSN 2078-5135
versión impresa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.105 no.7 Pretoria jul. 2015
http://dx.doi.org/10.7196/SAMJNEW.7881
RESEARCH
Intracranial suppuration: Review of an 8-year experience at Umtata General Hospital and Nelson Mandela Academic Hospital, Eastern Cape, South Africa
M A Anwary
MMed (DRad); Department of Radiology, Walter Sisulu University, Nelson Mandela Academic Hospital and Umtata General Hospital, Mthatha, Eastern Cape, South Africa
ABSTRACT
BACKGROUND: Intracranial suppuration (ICS) is a life-threatening condition caused by various disease processes and consisting of brain abscess and extradural and subdural empyema. The major causes have changed over the decades. To the author's knowledge, the incidence of ICS in South Africa (SA) has not been established.
OBJECTIVE: To determine the incidence of ICS, overall and according to age and gender, and to identify the source and distribution of ICS.
METHOD: The archive of the radiology departments at Umtata General Hospital and Nelson Mandela Academic Hospital in the Transkei region, Eastern Cape Province, SA, was searched retrospectively for computed tomography (CT) reports of patients diagnosed with ICS. Cases in which the CT images, patients' clinical information and CT reports were available for an uninterrupted period of at least 1 year were included.
RESULTS: Five time frames were established, encompassing 8 years of data. The first time frame established an incidence of ICS of 1/100 000/year for the Transkei region. All the time frames were utilised to determine the incidence according to gender and age, and the source and distribution of ICS. The incidence of ICS was higher among males than females, and highest in the age groups 0 - 10 and 11 - 20 years. A seasonal variation in the incidence of sinusitis- and meningitis-related ICS was noted. Numbers of cases declined during the last 3 years of the study period.
CONCLUSION: Sinusitis, head trauma, ear infection and meningitis were the major sources of ICS. A pulmonary source was not a major feature. In the last 4 years, trauma became the commonest source of ICS. A steady decline in ear infection- and meningitis-related ICS was noted.
Intracranial suppuration (ICS) is a serious and life-threatening complication of various disease processes. It consists of extradural empyema (EDE), subdural empyema (SDE) and brain abscess. The main causes of ICS are paranasal sinusitis, ear infection, head injury, meningitis, and metastatic spread from distant foci.[1-5] In some cases, the source of ICS is not identifiable.[1-5]Computed tomography (CT) is the main imaging modality used in the diagnosis of ICS. With adequate clinical information and CT examination an accurate diagnosis can be achieved in the majority of cases, magnetic resonance imaging being reserved for cases in which a conclusive diagnosis cannot be made with CT.
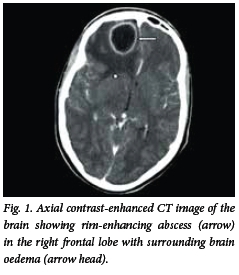
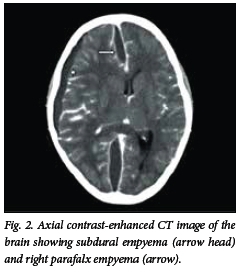
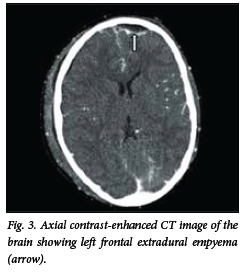
Brain abscess appears on the CT scan as an isodense or hyperdense ring, typically of uniform thickness with a hypodense centre (Fig. 1). Following intravenous injection of contrast medium, mild to intense ring enhancement of the abscess wall is seen. A hypodense area may be present surrounding the abscess, and represents vasogenic oedema. The presence of ventriculitis is seen as enhancement of the ependyma. On the CT scan, SDE appears as a hypodense or isodense crescentic or lenticular area adjacent to the inner table of the skull (Fig. 2). On a contrast-enhanced CT scan, enhancement of the medial rim is usually seen. EDE appears as a biconvex hypodense area between the inner table of the skull and the brain (Fig. 3). Contrast-enhanced scans show a well-demarcated rim of enhancement representing the dura.
In July 1992, the then government of the Republic of Transkei purchased and installed the first and the only CT scanner in Transkei at Umtata General Hospital, to serve a population of approximately three million people. Umtata General Hospital, being the only referral and teaching hospital in Transkei, received patients from 32 peripheral hospitals. The referral system was such that all patients needing specialist care were first referred to Umtata General Hospital for diagnosis and management and only then referred to other South African (SA) hospitals as needed. Umtata General Hospital therefore received most, if not all, of its patients from a well-defined and largely rural geographical area.
Since the incidence of ICS in SA has not been established, this study sought to determine the overall incidence of ICS as well as the incidence according to age and gender. The sources and distribution of ICS were also identified.
Methods
This was a retrospective study. The archive of the radiology departments at Umtata General Hospital and Nelson Mandela Academic Hospital in the Transkei region, Eastern Cape Province, SA, was searched for CT scan reports of the patients diagnosed with ICS. Cases in which the CT images, patients' clinical information and CT reports were available for an uninterrupted period of at least 1 year were included in the study. The following time frames fitted the latter criterion: (i) June 1993 - May 1994; (ii) January 1996 - December 1996; (iii) January 2001 -December 2001; (iv) June 2005 - May 2006; and (v) July 2007 - June 2011.
Time frame (i) (June 1993 - May 1994) represents patients from the entire Transkei region serviced by Umtata General Hospital and 32 peripheral hospitals. After May 1994, Transkei became incorporated into SA, with the result that some patients presented at non-Transkei hospitals and clinics. Also, following the formation of the five regions of the Eastern Cape Province in 1995, the peripheral hospitals of Transkei could refer patients to Frere Hospital (East London), Cecilia Makiwane Hospital (Mdantsane) and Queenstown Hospital, the province's designated referral hospitals. Time frames (ii) - (iv) therefore represent the post-regionalisation period and a smaller referral base consisting of Umtata General Hospital and 22 peripheral hospitals. The overall incidence of ICS for Transkei was therefore determined using the first time frame. The other incidences, causes and distribution of ICS were determined using a combination of all the time frames.
All consecutive cases diagnosed as brain abscess, extradural and subdural empyema, or combinations thereof were included in the study. Cases in which a conclusive CT diagnosis was not possible were excluded. The CT images for all the cases were stored on magnetic tape, a magnetic optic disc or a picture archiving system. The clinical information on all the patients was copied and archived.
Results
Incidence
During time frame June 1993 - May 1994, Umtata General Hospital and 32 peripheral hospitals served a population of approximately 3 million; 31 cases of intracranial suppuration were diagnosed, giving an incidence of approximately 1/100 000/year.
Presentation
During the five time frames, consisting of 8 years, 145 patients with ICS were diagnosed, of whom 93 were male and 52 female. Their ages ranged from 1 month to 61 years (Table 1); 75% of the patients were in the age group 0 - 20 years and 46% in the age group 11 - 20 years.
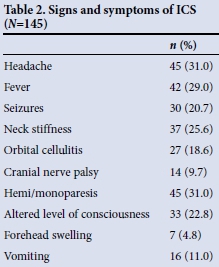
Signs and symptoms
The common presenting signs and symptoms are shown in Table 2. The most common symptoms were headache (31%) and vomiting (11%). The most common signs included fever (29%), hemi/monoparesis (31%), neck stiffness (26%) seizures (21%), altered level of consciousness (23%) and orbital cellulitis (19%). Of 27 patients who had orbital cellulitis, sinusitis was the cause in 23. Of the 45 presenting with hemi/ monoparesis, 25 had brain abscess, 19 empyema and 1 a combination of abscess and empyema. There were 14 cases of cranial nerve palsy, of which 10 were caused by abscess and 4 by subdural empyema.
Sources of ICS
The sources of ICS are listed in Table 3. The most common sources were sinusitis (n=38), head trauma (n=33), ear infection (n=22) and meningitis (n=24). In 15 cases (10%) the source of sepsis was not found.
The incidence of sinusitis-related ICS as a percentage of all the cases per year remained stable within a range of 21 - 36% ,while a general increase in head trauma-related ICS was noted (range 4.5 - 44%). During the last 4 years of the study period, only 4 cases of ear infection-related and 5 cases of meningitis-related ICS were seen.
The sources of ICS according to age are listed in Table 4. Common sources in the first decade of life were meningitis, sinusitis, trauma and ear infection. In the second decade, sinusitis was the commonest cause, followed by ear infection, trauma and meningitis. Head trauma was the commonest source of ICS above the age of 20 years, 54% of trauma-related ICS occurring in the age group 0 - 20 years and 79% in the age group 0 - 30 years. Unknown causes of ICS were more frequent in older patients (8 of the 35 patients aged >20 years as opposed to 7/110 patients aged <20 years), i.e. the source of ICS tended to become more obscure with older patient age.
Of the known common causes of ICS, sinusitis and trauma were more prevalent in males than in females (Table 5); in fact, trauma-related ICS was seven times more common in males, in keeping with the high incidence of trauma in this group.[9] Sinusitis-related ICS was twice as common in males than in females, and meningitis was a more common source in females than in males.

Types of ICS (Table 6)
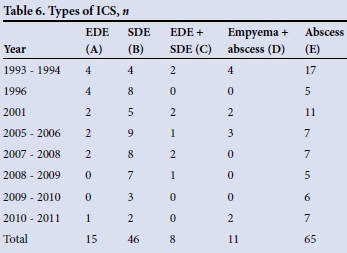
During the review period, there were 65 cases of abscess, 15 of EDE, 46 of SDE, 8 combinations of EDE and SDE, and 11 combinations of abscess and empyema. In total there were 76 cases of abscess (D + E in Table 6) and 80 cases of empyema (A + B + C + D). SDE (B + C) was more than twice as common as EDE (A + C). There were 16 patients with multiple abscesses.
Sources of abscess
The common sources of abscess were trauma (n=25), meningitis (n=17) and ear infection (n=11). There were 15 cases of ICS in which the source of sepsis could not be determined; of these, 12 were abscesses and 3 empyemas. In 16% of the abscesses the source of sepsis could not be found, while this was the case in only 3% of the empyemas. This finding highlights the occult nature of some of the sources of brain abscess.
Sources of empyema
The common sources of EDE were sinusitis (n=13) and ear infection (n=8). The common sources of SDE were sinusitis (n=29), trauma (n=10), meningitis (n=9) and ear infection (n=5).
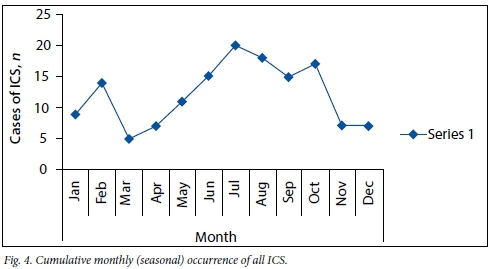
Cumulative monthly occurrence of ICS
During the process of data analysis, it was found that the incidence of ICS was higher during certain months. Fig. 4 shows the cumulative monthly occurrence of ICS from all causes. The incidence was highest from June through to October, accounting for 58% of the cases. Further analysis of the causes of ICS during this period revealed that sinusitis was a major contributor.
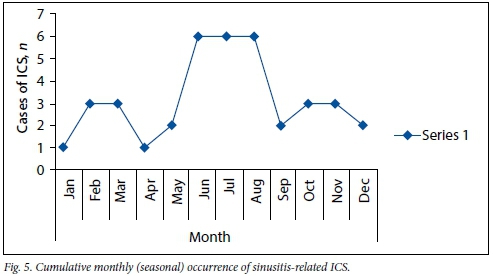
The cumulative monthly occurrence of sinusitis-related ICS (Fig. 5) shows that the incidence was highest during the winter months of June, July and August; 47% of all sinusitis-related ICS occurred during these months.
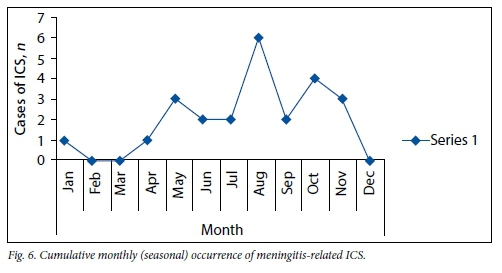
The cumulative monthly occurrence of meningitis-related ICS (Fig. 6) revealed a relatively low incidence from December through to April. Of the 22 cases, only 2 were seen during these summer and autumn months. Other causes of ICS did not vary significantly in seasonal occurrence.
Distribution of ICS
Abscesses commonly occurred in the frontal (n=27), temporal (n=21) and parietal lobes (n=15) and the cerebellum (n=15). Of the 15 cerebellar abscesses, 9 were caused by ear infection. The major sources of frontal lobe abscess were trauma (n=11), meningitis (n=7) and sinusitis (n=5). Temporal lobe abscesses were related to meningitis (n=7), trauma (n=5) and ear infection (n=3). Parietal lobe abscesses were mainly trauma related (n=9). The abscesses of unknown source showed no predilection for any particular area.
EDE was predominantly seen in the frontal area (n=18), followed by the temporal area (n=7) and posterior fossa (n=5). Of the 18 cases of frontal EDE, 13 were related to sinusitis. There were 5 EDEs in the posterior fossa, all caused by ear infection.
SDE predominantly occurred in the frontal (n=43), temporal (n=28), parietal (n=24) and parafalx areas (n=38), and was linked to sinusitis (n=29), trauma (n=10) and meningitis (n=9). The parafalx empyemas were mainly caused by sinusitis (n=19), trauma (n=8) and meningitis (n=5).
Discussion
The incidence of 1/100 000/year for ICS in the Transkei region is similar to the incidences of 1.1/100 000/year in Olmstead County, Minnesota, USA[6] and 1/100 000/ year in Edinburgh, UK.[7] However, it is much higher than the incidences of 0.3/100 000/ year in Wimbledon, London, UK,[3] and 0.3 -0.5/100 000/year in Northern Ireland.[8]
The higher incidence of ICS in males than in females in this study is reflected in other SA studies, which showed ratios of 2:1[4]and 2.8:1.[1] The ratio was 2:1 in Merseyside, UK,[2] and 4:1 in Olmstead County, USA.[6] Gender analysis of trauma-related ICS revealed a male bias of 7:1, in keeping with the higher incidence of head trauma among males.[9]
This study revealed a high incidence of ICS in the first two decades of life, accounting for 75% of all the cases over the study period. Other SA studies found that 52% of cases were in this age group[1] and that 23% were in the age group 0 - 13 years.[5] In Edinburgh, 43% of cases were in the age group 10 - 29 years.[2]
Common signs and symptoms in the studies reviewed vary substantially, and include headache (26 - 82%), fever (26 - 51%), focal neurological deficit (70%), neck stiffness (36 - 45%) and seizures (25 - 34%).[1-3]
The major causes of ICS in this study were sinusitis (26%), head trauma (23%), ear infection (15%) and meningitis (16%), which is similar to a study by Alegria et al.[1] in Johannesburg, SA, during January 1977 - December 1979 that showed sinusitis (25%), trauma (24%) and ear infection (20%) to be the major sources of ICS. These figures are comparable to ours despite a gap of more than 14 years between the two studies. The higher incidence of ICS due to meningitis in our study appears to be related to the increased incidence of tuberculosis.
The increased incidence of sinusitis-related ICS in June, July and August is in keeping with the fact that sinusitis is commonest during winter.
Numbers of cases of ICS in this study fell from 31 in 1993 - 1994 to 17 in 1996 as a result of a smaller referral base. The number of cases seen then stabilised at 22 for 2001 and 2005 - 2006, but a further decline was seen to 9 cases in 2009 - 2010, with 12 in 2010 - 2011. The decrease may reflect the general improvement in healthcare delivery in the Transkei region. A significant decrease in meningitis- and ear infection-related ICS was noted, with one case of meningitis-related and two cases of ear infection-related ICS seen during the last 2 years of the study. The incidence of sinusitis-related ICS was stable, while trauma-related ICS showed a steady increase. Further follow-up is required to confirm these trends.
References
1. Alegria C, Lipschitz R, Zwonninkoff G. Intracranial suppuration: A review of 79 cases seen at Baragwanath hospital over 3 years. S Afr J Surg 1982;20(1):25-35. [ Links ]
2. Bradley PJ, Shaw MDM. Three decades of brain abscess in Merseyside. J R Coll Surg Edinb 1983;28(4):223-229. [ Links ]
3. Miller ES, Dias PS, Uttley D. CT scanning in the management of intracranial abscess: A review of 100 cases. Br J Neurosurg 1988;2(4):439-446. [http://dx.doi.org/10.3109/02688698809029597] [ Links ]
4. Danziger A, Price H, Schecter MM. An analysis of 113 intracranial infections. Neuroradiology 1980;19(1):31-34. [ Links ]
5. Snyman H, Kruger P, van den Heever CM. Intracranial sepsis: A microbiological review. S Afr J Surg 1986;24(2):78-80. [http://dx.doi.org/10.1093/infdis/154.3.399] [ Links ]
6. Nicolosi A, Hauser WA, Beghi E, Kuland LT. Epidemiology of central nervous system infection in Olmstead County, Minnesota, 1950-1981. J Infect Dis 1986;154(3):399-408. [http://dx.doi.org/10.1093/infdis/154.3.399] [ Links ]
7. Small M, Dale BAB. Intracranial suppuration 1968-1982: A 15-year review. Clin Otolaryngol 1984;9(6):315-321. [http://dx.doi.org/10.1111/j.1365-2273.1984.tb01514.x] [ Links ]
8. McClelland C, Craig BF, Crockard HA. Brain abscesses in Northern Ireland: A 30 year community review. J Neurol Neurosurg Psychiatry 1978;41(11):1043-1047. [http://dx.doi.org/10.1136/jnnp.41.11.1043] [ Links ]
9. Nell V, Brown DSO. Epidemiology of traumatic brain injury in Johannesburg: II. Morbidity, mortality and etiology. Soc Sci Med 1991;33(3):289-296. [http://dx.doi.org/10.1016/0277-9536(91)90363-H] [ Links ]
 Correspondence:
Correspondence:
M A Anwary
radiologynmah@gmail.com
Accepted 8 November 2014.














