Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SAMJ: South African Medical Journal
versión On-line ISSN 2078-5135
versión impresa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.105 no.7 Pretoria jul. 2015
http://dx.doi.org/10.7196/SAMJNEW.7820
RESEARCH
The impact of highly active antiretroviral therapy on the burden of bacterial lower respiratory tract infections in children
K R de CamposI; D D GrangaII; S OlorunjuIII; R MasekelaIV
IMB ChB, MMed (Paed), Dip Allerg (SA); Department of Paediatrics and Child Health, Faculty of Health Sciences, University of Pretoria, South Africa, and Steve Biko Academic Hospital, Pretoria
IIMB ChB, MMed (Paed); Department of Paediatrics and Child Health, Faculty of Health Sciences, University of Pretoria, South Africa, and Steve Biko Academic Hospital, Pretoria
IIIPhD; Biostatistics Unit, Medical Research Council, Pretoria, South Africa
IVMB BCh, MMed (Paed), Dip Allerg (SA), Cert Pulmonol (SA) Paed, FCCP, PhD; Department of Paediatrics and Child Health, Faculty of Health Sciences, University of Pretoria, South Africa, and Steve Biko Academic Hospital, Pretoria; Department of Paediatrics and Child Health, School of Clinical Medicine, College of Health Sciences, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
ABSTRACT
BACKGROUND: Respiratory diseases are common and associated with significant morbidity and mortality in children.
OBJECTIVE: To evaluate the prevalence and outcome of bacterial lower respiratory tract infections (LRTIs) in HIV-infected and uninfected children at a primary level hospital.
METHODS: A cross-sectional descriptive study of children aged 6 months - 18 years was conducted. Recruitment included HIV-positive children who had been on highly active antiretroviral therapy (HAART) for at least 6 months. A comparator group of HIV-negative children admitted with bacterial pneumonia was included. Laboratory data collected included CD4+ T-cell counts, HIV viral load and C-reactive protein (CRP). Data collected in both groups included demographic data, immunisation status, zinc supplementation, previous LRTIs, environmental exposures and treatment.
RESULTS: Fifty-nine HIV-infected and 20 uninfected children were enrolled. The HIV-positive children were older, with a mean age of 107.2 (standard deviation 50.0) months v. 12.0 (5.8) months (p<0.005). The HIV-infected group had a mean CD4 percentage of 31.5%, and had had an average of 3.9 visits for bacterial LRTIs. All were treated with amoxicillin with no complications. In the HIV-uninfected group, cough and rapid breathing were the most common presenting symptoms, and the mean CRP level was 463.0 mg/L. The mean hospital stay was 4 days.
CONCLUSION: HAART is effective in reducing the burden of LRTIs in HIV-positive children, even when the diagnosis is delayed. Cough and fast breathing are still the most reliable presenting symptoms of pneumonia. The majority of children still respond to amoxicillin as first-line therapy, with low complication rates.
Respiratory diseases are common in children and carry a significant burden of morbidity and mortality in this age group worldwide.[1] Lower respiratory tract infections (LRTIs) are defined as infections that affect airways below the epiglottis. The term is often used as a synonym for pneumonia, which is defined as inflammation of the lung parenchyma with cough, dyspnoea and indrawing of the chest wall the most common presenting symptoms and signs.[2]
Healthy children are vulnerable to pneumonia when the immune system is weakened by factors such as immunosuppression, malnutrition, measles, overcrowded homes, parental smoking or indoor pollution. Several steps have been established by the World Health Organization (WHO) to reduce mortality and morbidity due to pneumonia. These include immunisation, promotion of adequate nutrition (including breastfeeding and zinc intake), reduction of indoor air pollution, and implementation of the Integrated Management of Childhood Illness (IMCI) programme.[3] Pneumonia currently accounts for 20% of deaths of children under 5 years of age in developing countries every year, and remains an important condition in HIV-infected children.[4]
An estimated 330 000 children acquired HIV infection in 2011, and more than 90% of these lived in sub-Saharan Africa.[5] In South Africa (SA), between 410 000 and 520 000 children aged 0 - 14 years are living with HIV.[6] Of the approximately 2.1 million children who are infected with HIV type 1, more than 80% will develop a respiratory illness at some stage during the course of their disease.[7]
The advent of highly active antiretroviral therapy (HAART) has changed the natural progression of the disease, reducing viral replication, increasing the number of CD4 lymphocytes, and thus re-establishing host defences and improving survival.[8] Nevertheless, even on HAART children remain more vulnerable to infections than their healthy uninfected peers.[9] A reduction in the rate of opportunistic infections and hospitalisations in adults with AIDS after 6 - 12 months of HAART intervention is well documented.[10] Decreases in hospitalisations and deaths result in a substantial reduction in the healthcare costs associated with infection. The Children with HIV Early Antiretroviral Therapy Trial showed that early diagnosis and early antiretroviral therapy (ART) reduced early infant mortality by 76% and HIV progression by 75%.[11] Worldwide, very few studies on the impact of HAART on vertically infected children and adolescents have been done.[10]
The other major intervention to reduce pneumonia-related morbidity and mortality among all children is implementation of preventive strategies. Routine immunisations against Streptococcus pneumoniae, Haemophilus influenzae and varicella are safe and effective even in HIV-infected children, in whom the primary immunological response is inferior and there is faster decay in immunological memory.[3] The role of nutritional inventions, such as exclusive breastfeeding and zinc supplements, in the prevention of pneumonia among HIV-infected children needs to be explored more thoroughly.[11,12]
Objective
The primary aim of this study was to assess the prevalence of bacterial LRTIs in HIV-infected and uninfected children. The secondary aim was to assess the outcome of bacterial LRTIs in infected and uninfected children managed at a primary level hospital.
Methods
It was hypothesised that there is no difference in the rate of LRTIs in HIV-positive children on HAART compared with HIV-negative children.
A cross-sectional descriptive study of children aged 6 months - 18 years was conducted at Tshwane District Hospital (TDH), Pretoria, SA, from January 2014 to September 2014. Two cohorts of children were recruited: a group of HIV-infected children who had been on HAART for at least 6 months, and a comparator group consisting of HIV-negative children admitted to the inpatient paediatric ward with a diagnosis of bacterial pneumonia.
For both groups of children, data collected included demographic information, number of healthcare visits for LTRIs in the past, number of antibiotic courses given for LRTIs, immunisation status (confirmed by assessment of the Road to Health card (RTHC)), documented zinc supplementation on the RTHC, and finally exposure to biomass fuels and environmental tobacco smoke. For the HIV-positive group, data collected included an assessment of HIV stage, which included CD4+ T-cell counts, HIV viral load and duration of HAART.
Patients were excluded if their HIV status was unknown or if consent could not be obtained for HIV testing.
Statistical analysis
Data analysis was performed using Stata 12 (StataCorp LP, 2011; Statistical Software, USA). Summary statistics for all variables were done. Fisher's exact test was used for assessing the association between categorical variables in the HIV-infected and uninfected children, and a two-sample independent f-test for proportions for comparisons of proportions of patients, both HIV-infected and uninfected, who had records of antibiotic therapy, zinc supplementation and immunisation. Similarly, a two-sample f-test was used for gender comparisons in the HIV-uninfected group. Testing was done at the 0.05 level of significance.
Results
A total of 59 HIV-infected children were recruited. Of 623 children screened for the HIV-uninfected group, only 20 met the inclusion criteria. The majority of children who were screened had either bronchiolitis or asthma.
The children in the HIV-uninfected group were younger (mean age 12.0 (standard deviation (SD) 5.8) months) than the HIV-infected children (mean age 107.2 (50.0) months) (p<0.005).
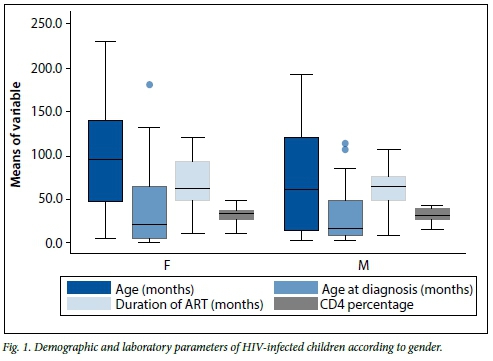
The majority of the HIV-infected children had been diagnosed after the age of 2 years, with most having been on HAART for over 5 years and having immune restoration with normal CD4+ T-cell counts (Table 1). When all the demographic and clinical variables in the HIV-infected group of children were compared, there was no statistically significant difference in any of the variables when comparisons were performed according to gender (all p>0.05) (Fig. 1). None of the HIV-infected patients received oxygen therapy, as they were seen and managed at an outpatient department or local clinics, with only one child requiring admission. Thirty-one (52.5%) of these children received antibiotics for LRTIs; only one had complicated pneumonia. None of these HIV-infected children had a record of zinc supplementation. The majority of the HIV-infected group (66.7%) also did not have a complete immunisation record.
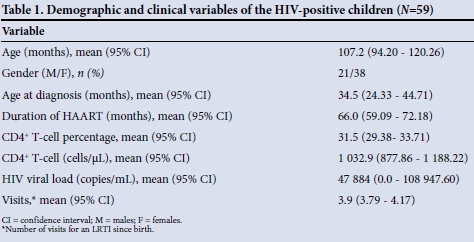
Twelve (20.3%) of the HIV-infected children had had five visits to the TDH clinic for an LRTI, and 15.3% had had three visits. Amoxicillin was the antibiotic of choice in 98.3% of cases. One patient had only received erythromycin.
Of the HIV-uninfected children who were admitted with an LRTI and screened during the study period, only 3.2% had bacterial pneumonia. The gender distribution was unbalanced, with 60.0% males, and the average age of the children was 12 months (Table 2). There was no difference in mean age at admission when the males and females were compared (13.87 months (95% confidence interval (CI) 9.62 - 18.12) v. 10.75 months (95% CI 6.84 - 14.66), respectively) (p=0.233). The most common presenting symptoms of pneumonia were cough, found in all children (100.0%), fast breathing (n=16, 80.0%) and chest indrawing (n=14, 70.0%). More females than males presented with chest indrawing (n=6 (33.3%) v. n=5 (25.0%)), but this was not statistically significant (p=0.41). Of the children with pneumonia, five required oxygen therapy. The mean CRP level was 463.0 mg/L, with no significant difference in CRP levels between males and females (450.8 mg/L (95% CI 327.7 - 573.9) v. 481.2 mg/L (95% CI 158.1 - 804.3), respectively) (p=0.841). The average number of admission days was 4.4, with no difference in number of days when the males and females were compared (p=0.663). Intravenous ampicillin was the first-line antibiotic of choice, with only 12.5% receiving ampicillin and amikacin and only one patient initiated on second-line therapy (ceftriaxone) owing to failed first-line treatment.
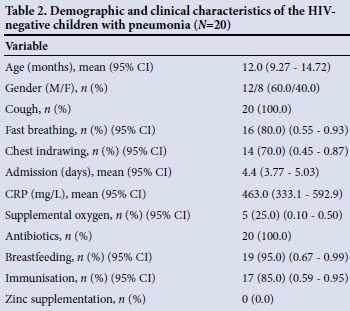
Exposure to biomass fuels was low in this study population (only 7.0%), the majority of the children's homes having electric stoves. No children were exposed to tobacco smoke. There was no record of zinc supplementation in any of the children's RTHCs. Breastfeeding rates were high, with 95.0% of all children being breastfed. Overall, 85.0% of the children were fully immunised, the proportion being higher among males than females (91.7% v. 75.0%), although this did not reach statistical significance (p=0.085).
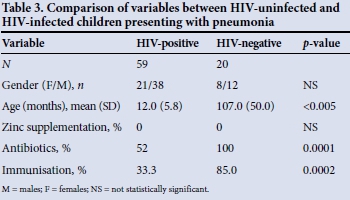
When the HIV-uninfected and HIV-infected groups were compared, all the HIV-uninfected children had been treated with antibiotics as opposed to 52.5% of the HIV-infected group (p<0.005). There was no documented record of zinc supplementation in either group. Immunisation rates differed between the groups, with more HIV-positive children than HIV-negative children having an incomplete record (62.0% v. 15.0%); this was statistically significant (p=0.002).
Discussion
This study shows that diagnosis of HIV infection and initiation of HAART may still be delayed in children; despite this, however, once HAART is initiated the burden of bacterial LTRIs is low, with children experiencing an average of less than one bacterial infection per year in the first 8 years of their lives. Admission rates for pneumonia were also very low, with no serious sequelae. Of concern are relatively low clinic attendance rates, with two-thirds of HIV-infected children having an incomplete immunisation record.
For the HIV-uninfected group, viral LRTIs were the most common cause for admission, with children who presented with bacterial LRTIs being younger than 2 years and typically having uncomplicated pneumonia with good outcomes. Cough and fast breathing remain the major presenting symptoms for bacterial pneumonia. Penicillin-based antibiotics were the first-line therapy in most cases, with the average duration of treatment being less than 5 days. Immunisation and breastfeeding rates were high and biomass exposures low.
HAART improves survival in HIV-infected children. The majority of HIV-infected children in the current study had been on HAART for over 5 years and had a normal CD4+ T-cell count and a suppressed HIV viral load. This may account for the low levels of recorded bacterial LRTIs and low complication rates. Sanchez et al.[13] found that HAART results in a lower risk of death at 5 years' follow-up. The impact of ART on lung health of HIV-infected persons is not well understood. Systemically, treatment with ART decreases HIV replication, immune activation and chronic inflammation and increases CD4+ T-lymphocyte counts. Within the alveolar space, ART decreases the pulmonary HIV viral load and decreases pulmonary inflammatory responses.[8] The present study confirms the effectiveness of HAART as the best intervention for the prevention of pneumonia, and consequently of hospitalisations and deaths, in children and adolescents living with HIV/AIDS. This is supported by the findings of Candiani et al.[10]
According to the WHO, pneumonia accounts for 19% of deaths of children aged under 5 and for 4% of neonatal deaths worldwide.[14] In SA, the mortality rate from pneumonia in children increased from 21/1 000 to 103/1 000 between 2003 and 2009. The annual incidence of pneumonia in children younger than 5 years of age in SA remains high, and pneumonia is one of the major causes of mortality in this age group.[15] Determining the causation of bacterial pneumonia in children is difficult, as specimens for culture are difficult to obtain and often yield negative results. For this reason several studies have advocated the use of empirical treatment, since S. pneumoniae is the most common bacterial pathogen identified in children aged 4 weeks and older in developing countries. The role of S. pneumoniae in serious infections has decreased significantly since the introduction of the pneumococcal conjugate vaccine (PCV).[16,17] In SA the PCV7 vaccine was included in the immunisation schedule in the public sector (which caters for over 80% of the population) in 2009.[18] In the current study, the majority of HIV-negative children were under 5 years of age and therefore fell into the group that would have benefited from this vaccine.
Vaccination with PCV is a public health intervention to prevent pneumococcal disease, and it was licensed in the USA for use in children in 2000. This vaccine is suitable for use in infants and includes the most commonly identified serotypes, namely 4, 6B, 9V, 14, 18C, 19F and 23F.[19] Recently two new vaccines covering other serotypes such as 1 and 5, which are highly invasive and responsible for severe illness and hospitalisation in young children, have been implemented, i.e. PCV10 and PCV13.[20] Several studies on the PCV vaccine have shown that it is effective in reducing invasive pneumococcal disease and hopitalisations.[21] We have observed that the majority of children admitted with 'pneumonia' and screened had viral bronchiolitis, with very few having bacterial pneumonia.
Most guidelines suggest treatment with amoxicillin as first-line antibiotic therapy for community-acquired bacterial pneumonia.[22] In our cohort, amoxicillin was the antibiotic of choice for both groups, while the first choice for inpatient therapy for the HIV-negative group was ampicillin. The response to treatment was good, based on the short length of hospital stay and the lack of significant complications observed, probably because S. pneumoniae remains the most common bacterial pathogen causing community-acquired pneumonia.
Healthy children are vulnerable to pneumonia when their immune system is weakened. Factors involved include parental smoking and indoor pollution, which is a huge problem in Africa, where about 700 million people burn biomass fuels to provide energy to cook. Smith et al.[23] have demonstrated in a randomised controlled trial that biomass exposure is a significant risk factor for pneumonia. We found low levels of biomass exposure, which may explain the low levels of bacterial pneumonia found in our study.
Other preventive strategies to reduce pneumonia morbidity and mortality include the use of the IMCI programme. In SA, use of this programme at primary care level has been shown to be very effective for the diagnosis and management of pneumonia.[24] In the current study, cough and fast breathing were the most common presenting symptoms, as per the WHO definition of pneumonia, confirming the value of these clinical symptoms for the diagnosis of pneumonia at primary care level. Acute-phase reactants such as CRP have been extensively studied in the evaluation and prognostication of pneumonia. Various cut-points for diagnosis have been suggested, with values ranging between 300 and 560 mg/L.[25] Youssef et al.[26] demonstrated a correlation between a high CRP level (mean in their study 916.8 mg/L) and severity of pneumonia and the need for intensive care unit (ICU) admission. In the current study, the mean CRP level was 460 mg/L and none of the patients had a prolonged hospital stay or required ICU admission.
The value of zinc supplementation as an adjuvant therapy for pneumonia prevention is still not well established. Shah et al.[27] performed a randomised double-blind placebo-controlled study and concluded that there was no significant reduction in duration of hospital stay. The majority of children in the current study did not receive any zinc supplementation; this did not seem to impact upon the severity or number of LRTIs experienced by children in this cohort, although our numbers were small.
Study limitations
The limitations of this study were the small numbers of children, particularly in the HIV-uninfected group, as the majority of children admitted had a viral LRTI. The fact that the HIV-infected children were older makes it difficult to compare them with a younger HIV-uninfected group, as younger children generally experience more LRTIs.
Recommendations
Future randomised controlled trials focusing on the role of zinc supplementation on the prevention of bacterial LRTIs in both HIV-infected and uninfected children should be conducted. In the current study, the HIV-infected children were older and a follow-up study of the same cohort of children in 5 years' time would be interesting to assess the ongoing protective role of HAART and its impact on lung health in adolescence.
Conclusion
HAART is effective in reducing the burden of LRTIs in children, even when the diagnosis of HIV infection is delayed. Cough and fast breathing are the most reliable presenting symptoms for pneumonia. The majority of children respond to amoxicillin as first-line therapy.
References
1. Roth DE, Caulfield LE, Ezzati M, Black RE. Acute lower respiratory infections of childhood. www.who.int/bulletin/volumes (accessed 14 May 2013). [ Links ]
2. Shah N. Pneumonia: The forgotten killer. Pediatr Infect Dis 2010;11:145-157. [http://dx.doi.org/10.1016/S2212-8328(10)80004-9] [ Links ]
3. Greenwood B. A global action plan for the prevention and control of pneumonia. Bull World Health Organ 2008;86(5):322-2A. [http://dx.doi.org/10.2471/BLT.08.053348] [ Links ]
4. Sazawa S, Black R. Effect of pneumonia case management on mortality in neonates, infants and preschool children: A meta-analysis of community-based trials. Lancet Infect Dis 2003;3(9):547-556. [http://dx.doi.org/10.1016/S1473-3099(03)00737-0] [ Links ]
5. Global report UNAIDS on the global AIDS epidemic: 2012. www.unaidsorg (accessed 13 May 2013). [ Links ]
6. Averting HIV and AIDS. HIV and AIDS in South Africa. International HIV and AIDS charity from AVERT. www.avert.org/aidssouthafrica.htm (accessed 13 May 2013). [ Links ]
7. Jeena PM. Can the burden of pneumonia among HIV-infected children be reduced? Bull World Health Organ 2008;86(5):323A-333A. [http://dx.doi.org/10.2471/BLT.08.053223] [ Links ]
8. Crothers K, Thompson BW, Burkhardt K, et al. HIV-associated lung infections and complications in the era of combination antiretroviral therapy. Proc Am Thorac Soc 2011;8(3):275-281. [http://dx.doi.org/10.1513/pats.201009-059WR] [ Links ]
9. Gray DM, Zar HJ. Community-acquired pneumonia in HIV-infected children: A global perspective. Curr Opin Pulm Med 2010;16(3):208-216. [http://dx.doi.org/10.1097/MCP.0b013e3283387984] [ Links ]
10. Candiani TM, Pinto J, Cardoso CA, et al. Impact of highly active antiretroviral therapy (HAART) on the incidence of opportunistic infections, hospitalizations and mortality among children and adolescents living with HIV/AIDS in Belo Horizonte, Minas Gerais State, Brazil. Cad Saude Publica 2007;23(Suppl 3):S414-S423. [http://dx.doi.org/10.1590/S0102-311X2007001500009] [ Links ]
11. Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med 2008;359(21):2233-2244. [http://dx.doi.org/10.1056/NEJMoa0800971] [ Links ]
12. Fataki MR, Kisenge RR, Sudfeld CR, et al. Effect of zinc supplementation on duration of hospitalization in Tanzanian children presenting with acute pneumonia. J Trop Pediatr 2014;60(2):104-111. [http://dx.doi.org/10.1093/tropej/fmt089] [ Links ]
13. Sánchez JM, Ramos Amador JT, Fernándes de Miquel SR, et al. Impact of highly active antiretroviral therapy on the morbidity and mortality in Spanish human immunodeficiency virus-infected children. Pediatr Infect Dis J 2003;22(10):863-877. [http://dx.doi.org/10.1097/01.inf.0000091282.70253.5f] [ Links ]
14. Zar HJ, Jeena PM, Argent A, Gie R, Madhi SA. Diagnosis and management of community-acquired pneumonia in childhood: South African Thoracic Society guidelines. S AfT J Epidemiol Infect 2009;24(1):25-36. [ Links ]
15. Global action plan for prevention and control ofpneumonia (GAAP). www.unicef.org (accessed 14 May 2013). [ Links ]
16. Zar HJ. Prevention of HIV-associated respiratory disease in developing countries: Potential benefits. Int J Tuberc Lung Dis 2003;7(10):820-827. [ Links ]
17. Statistical Services South Africa. South Africa 2005. Millenium Development Goals Country Report. www.statssa.gov.za (accessed 14 May 2013). [ Links ]
18. Zar HJ, Madhi S. Pneumococcal conjugate vaccine - advancing child health in South Africa. S Afr J Child Health 2008;2(3):94-95. [ Links ]
19. Hausdorff WP, Bryant J, Paradiso PR, Siber GR. Which pneumococcal serogroups cause the most invasive disease: Implications for conjugate vaccine formulation and use, part 1. Clin Infect Dis 2000;30(1):100-121. [http://dx.doi.org/10.1086/313608]) [ Links ]
20. Gessner BD, Adegbola RA. The impact of vaccines on pneumonia: Key lessons from Haemophilus influenzae type b conjugate vaccine. Vaccine 2008;26(Suppl):B3-B8. [http://dx.doi.org/10.1016/j.vaccine.2008.04.013] [ Links ]
21. Von Gottberg A, de Gouveia L, Madhi S, et al. The impact of conjugate Haemophilus influenza type B (Hib) vaccine introduction in South Africa. Bull World Health Organ 2006;84(10):811-818. [http://dx.doi.org/10.2471/BLT.06.030361] [ Links ]
22. Mc Cracken GH. Diagnosis and management of pneumonia in children. Pediatr Infect Dis J 2000;19(9):924-928. [http://dx.doi.org/10.1097/00006454-200009000-00036] [ Links ]
23. Smith KR, McCraken JP, Weber MW, et al. Effect of reduction in household air pollution in childhood pneumonia in Guatamala: A randomized controlled trial. Lancet 2011;378(9804):1717-1726. [http://dx.doi.org/10.1016/S0140-6736(11)60921-5] [ Links ]
24. KZN IMCI Guidelines. Integrated Management of Childhood Illness. www.kznhealthgov.za/imci (accessed 14 May 2013). [ Links ]
25. Van Vugt SF, Broekhuizen BD, Lammens C, et al. Use of serum C reactive protein and PCT concentration in addition to symptoms and signs to predict pneumonia in patients presenting to primary care with acute cough: Diagnostic study. BMJ 2013;346:F2450. [http://dx.doi.org/10.1136/bmj.f2450] [ Links ]
26. Youssef HA, Nasseh S, Hafiz AH, Gawesh A. Evaluation of diagnostic and prognostic value of high sensitivity C reactive protein (hsCRP) in community acquired pneumonia. Egyptian Journal of Chest Diseases and Tuberculosis 2013;62(2):301-304. [http://dx.doi.org/10.1016/j.ejcdt.2013.05.011] [ Links ]
27. Shah GS, Dutta AK, Shah D, Mishra OP. Role of zinc in severe pneumonia: A randomized double blind placebo controlled study. Ital J Pediatr 2012;38:36. [http://dx.doi.org/10.1186/1824-7288-38-36] [ Links ]
 Correspondence:
Correspondence:
R Masekela
masekelar@ukzn.ac.za
Accepted 20 March 2015.














