Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SAMJ: South African Medical Journal
versión On-line ISSN 2078-5135
versión impresa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.105 no.6 Pretoria jun. 2015
http://dx.doi.org/10.7196/SAMJ.8788
RESEARCH
Paediatric dental chair sedation: An audit of current practice in Gauteng, South Africa
F BhamI; H PerrieII; J ScribanteIII; C-A LeeIV
IMB BCh, DA (SA); Department of Anaesthesiology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIMSc; Department of Anaesthesiology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIIMCur; Department of Anaesthesiology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IVMB ChB, FCA (SA), MMed (Anaesthesiol); Private practice, Johannesburg, South Africa
ABSTRACT
BACKGROUND: Procedural sedation and analgesia (PSA) is often required to perform dental procedures in children. Serious adverse outcomes, while rare, are usually preventable
OBJECTIVES: To determine the proportion of dental practitioners making use of paediatric dental chair PSA in Gauteng Province, South Africa, describe their PSA practice, and determine compliance with recommended safety standards
METHOD: A prospective, contextual, descriptive study design was used, with 222 randomly selected dental practitioners contacted to determine whether they offered paediatric dental chair PSA. Practitioners offering PSA were then asked to complete a web-based questionnaire assessing their practice
RESULTS: Of the 213 dental practitioners contacted, 94 (44.1%; 95% confidence interval 37 - 51) provided PSA to children. Most patients were 1 - 5 years old, although there were practices that offered PSA to infants. While most procedures were performed under minimal to moderate sedation, deep sedation and general anaesthesia were also administered in dental rooms. Midazolam was the most frequently used sedative agent, often in conjunction with inhaled nitrous oxide; 28.1% of PSA providers administered a combination of three or more agents. Presedation patient assessment was documented in 83.0% of cases, and informed consent for sedation was obtained in 75.6%. The survey raised several areas of concern regarding patient safety: 41.3% of dental practices did not use any monitoring equipment during sedation; the operator was responsible for the sedation and monitoring of the patient in 41.3%; 43.2% did not keep any recommended emergency drugs; and 19.6% did not have any emergency or resuscitation equipment available. Most respondents (81.8%) indicated an interest in sedation training
CONCLUSION: Paediatric dental chair PSA was offered by 44.1% of dental practitioners interviewed in Gauteng. Modalities of PSA provided varied between practices, with a number of safety concerns being raised.
Procedural sedation and analgesia (PSA) is generally safe, and is often necessary to facilitate dental procedures in children. If provided in dental rooms, PSA is cost-effective, avoiding the expenses generated by having to perform procedures in operating theatres. It allows the dentist to practise in the familiarity of his or her own rooms,[1] and overcomes the need to rely on the limited availability of anaesthetists. Dental chair PSA should not, however, be performed at the cost of patient safety. While serious adverse events, including death and permanent neurological injury, occur rarely, they are nearly always preventable. Tragically, these adverse outcomes have been reported in healthy children sedated for minor procedures, suggesting the need to adopt guidelines that could reduce the risk associated with PSA.[2]
A USA-based critical incident analysis of 118 reported serious adverse paediatric sedation events revealed that, while respiratory compromise was the initial event in over 80% of adverse events, regardless of whether a child was sedated in or out of hospital, a final outcome of death or permanent neurological injury occurred more frequently in an out-of-hospital than an in-hospital setting (92.8% of events v. 37.2%; p<0.001). The most common contributory cause was drug interactions, implicated in 46.3% of events. Drug overdose, inadequate monitoring, inadequate resuscitation, inadequate medical evaluation and premature discharge were also shown to be causes contributing to these events, some of which occurred at home or on the way to or from the facility (sedatives were sometimes being prescribed to give at home before leaving for the procedure). Reports like these led to the widespread development of guidelines for safe procedural sedation.[3]
The South African Society of Anaesthesiologists (SASA) published a guideline for the safe use of procedural sedation and analgesia for diagnostic and therapeutic procedures in children in 2010 (SASA PSA guideline).[4] The aim of the guideline is to provide a reference to enable all medical practitioners, including dentists, to act within a framework that ensures patient safety and to provide safe sedation, analgesia and anxiolysis in all environments. The guideline provides guidance on patient selection, recommended drugs and dosages, equipment, monitoring, documentation and discharge criteria.[4]
The World Health Organization (WHO) has identified the need for research aimed at improving patient safety as a priority, especially in developing and transitional countries.[5] No previously published data were identified on the percentage of dental practices in Gauteng utilising PSA and whether sedation practitioners are aware of the available SASA PSA guideline.[4] Comparing data with recommended safety standards may identify areas of concern and serve as a guide to developing measures that will enhance safety during paediatric dental chair sedation.
Objectives
To determine the proportion of dental practitioners making use of paediatric dental chair PSA in Gauteng, and to describe their practice and adherence to the SASA PSA guideline.[4]
Methods
Approval to conduct this study was obtained from the Human Research Ethics Committee (Medical) of the University of the Witwatersrand, Johannesburg.
A prospective, contextual, descriptive study design was used. The study population comprised qualified dental practitioners listed on the South African Dental Association website and practising in Gauteng. The names and contact numbers of 1 152 practitioners were available to the public on the website on 23 June 2012.[6] Two hundred and twenty-two of them were selected by simple random sampling and invited telephonically to participate in the study. The sample size was determined in consultation with a biostatistician and influenced by available resources (financial and time constraints) and the scope of the study. Practitioners who consented to participate and whose practice provided dental chair PSA to children up to 12 years old were then sent an introductory email with a link to a web-based questionnaire.
The questionnaire was designed using SurveyMonkey, a commercial online survey site. Before developing the questionnaire, the relevant literature was reviewed to identify the potential safety pitfalls in the field of paediatric dental chair PSA. The SASA PSA guideline[4] served as the main reference point for the development of the questionnaire (content validity), which assessed items in the following categories: the professional category of the person responsible for administering PSA; modalities of sedation administered; awareness of the SASA PSA guideline;[4] training of the sedationist; patient selection; monitoring; and emergency equipment and drugs. The questionnaire was developed in consultation with three experts in the field (face validity).
Confidentiality and anonymity of information were ensured, as all responses were uploaded anonymously onto the SurveyMonkey website for analysis and the researchers were the only people with access to this database by way of a user name and password. A reminder was emailed to all participants 2 weeks after the initial email to thank those practitioners who had completed the questionnaire and to serve as a reminder to those who had not yet done so.
The following definitions were used in this study, consistent with those used in the SASA PSA guideline:
- Minimal sedation and anxiolysis. The patient responds normally to verbal commands. Cognitive function and co-ordination may be impaired, but ventilation and cardiovascular function are unaffected.[4]
- Moderate sedation and analgesia. Depression of consciousness during which the patient can respond purposefully to verbal commands, either alone or accompanied by light tactile stimulation. The patient is able to maintain a patent airway and spontaneous ventilation.[4]
- Deep sedation and analgesia. The patient cannot easily be roused, but may respond purposefully following repeated or painful stimulation. Assistance may be required to maintain a patent airway and spontaneous ventilation may be inadequate. Cardiovascular function is usually maintained.[4]
- General anaesthesia. Patients cannot be roused, even by painful stimulation. Patients require assistance in maintaining a patent airway and positive-pressure ventilation may be required. Cardiovascular function may be impaired.[4]
Descriptive statistics using frequencies and percentages were used to analyse the data. A 95% confidence interval (CI) was reported for the proportion of dental practitioners interviewed who used paediatric dental chair PSA.
Results
Two hundred and twenty-two dental practitioners were contacted telephonically between April and May 2013. Nine were excluded from the study because they were no longer in clinical practice. Data analysis therefore included 213 dental practitioners, comprising 195 general dentists and 18 specialists (6 orthodontists, 5 periodontists, 4 maxillofacial surgeons and 3 prosthodontists).
Ninety-four of the 213 dental practitioners interviewed offered paediatric dental chair PSA as part of their routine practice (44.1%; 95% CI 37 - 51). The participant information letter containing a link to the questionnaire was sent to 93 of the 94 practitioners, as one respondent did not have an email address. Of the 93 questionnaires issued, 52 (55.9%) were returned to the SurveyMonkey database for further analysis. Certain questions in the questionnaire were omitted by some respondents, with no clear pattern of omissions emerging.
Table 1 presents the professional categories of PSA providers. Of the 48 respondents to this question, 22 indicated that they were primarily responsible for PSA administration in their practice (45.8%). Of these, 12 (54.6%) had received sedation training and 4 (20%) were aware of the SASA PSA guideline.[4] Thirty-six of 44 respondents (81.8%) were interested in attending a sedation course.
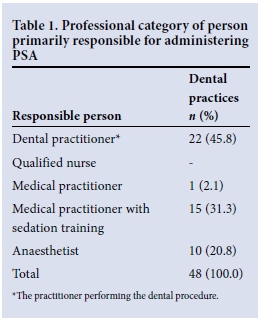
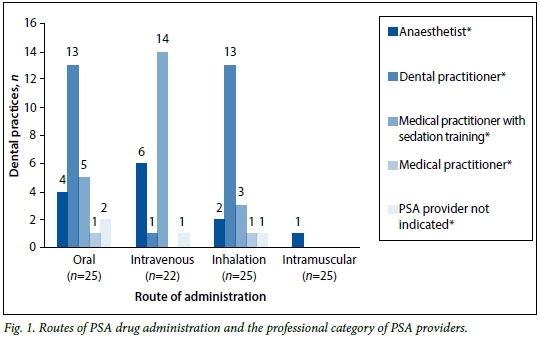
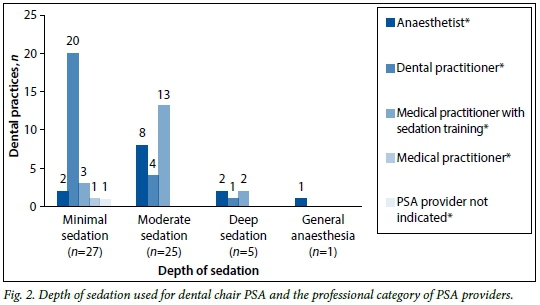
Forty-eight respondents indicated the route(s) of drug administration and depth(s) of sedation administered in their rooms. These results are broken down into professional categories of PSA providers and illustrated in Figs 1 and 2, respectively. Drugs were most commonly given orally (n=25, 52.1%), although the intravenous (n=22, 45.8%), inhalational (n=20, 41.7%), and intramuscular (n=1, 2.1%) routes were also used. Twenty-seven practitioners (56.3%) performed procedures under minimal sedation and 25 (52.1%) utilised moderate sedation. Deep sedation (n=5, 10.4%) and general anaesthesia (n=1; 2.1%) were provided in fewer dental rooms.
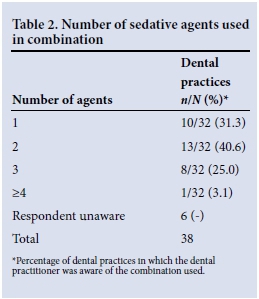
Thirty-two dental practitioners indicated the number of agents used in combination during PSA. Table 2 presents these data. Midazolam, nitrous oxide (N2O) and propofol were the most popular agents used for sedation, used in 68.8%, 39.6% and 27.1% of practices, respectively.
The 1 - 5- and 6 - 8-year-old age groups were most commonly sedated (76.6% and 74.5% of 47 dental practices, respectively), followed by the 9 - 12-year-old age group (20 practices, 42.6%). Two dental practitioners performed procedures under PSA in infants.
Presedation assessment was conducted in 83.0% of practices, with 75.6% obtaining informed consent prior to providing PSA.
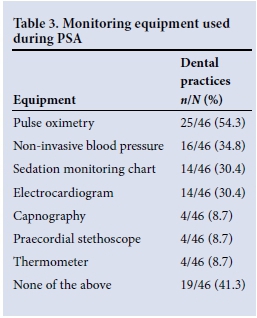
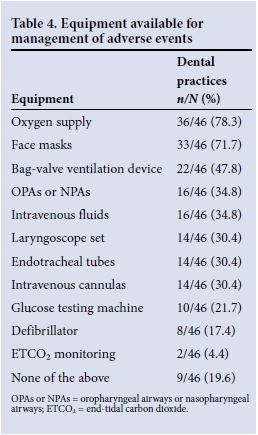
Forty-six respondents indicated the monitoring equipment used during PSA and the emergency equipment available for management of any adverse events. Tables 3 and 4 present these data. The dental practitioner was the person responsible for the sedation and monitoring of children in 41.3% of practices. No monitoring equipment was used in 41.3% of practices. Oxygen was available in 36 practices (78.3%), with 14 practices (30.4%) having emergency airway equipment.
Of the 44 dental practitioners who indicated which emergency drug(s) were available during PSA procedures, 19 (43.2%) did not keep any emergency drugs, 25 (56.8%) kept adrenaline, 14 (31.8%) stocked atropine, 8 (18.2%) had succinylcholine, 6 (13.6%) had flumazenil, and 5 (11.4%) kept naloxone. Flumazenil was stocked by 5 (15.2%) of the 33 respondents who used midazolam and by 1 (10.0%) of the 10 diazepam users. Naloxone was stocked by 1 of the 5 respondents who used alfentanil (20.0%), but by none of the practices in which fentanyl (2), pethidine (1) or tilidine (1) were administered.
Of the 20 respondents who were primarily responsible for providing PSA in their rooms, 18 (90.0%) had received Basic Life Support training and 2 (10.0%) had Advanced Paediatric Life Support certification.
A staffed recovery area was available in 60.5% of practices.
Apart from one report of an allergic reaction, no serious adverse events or complications were reported in this survey.
Discussion
While no significant adverse events or complications were reported during this audit of current practice in Gauteng, some of the findings raise concerns about the safety of children being sedated for procedures in the dental chair.
In this study, 45.8% of PSA was provided by the dental practitioner, who was also the operator. Patients receiving PSA were being monitored by the dental practitioner in 41.3% of practices. The SASA guideline states that even for basic sedation (which includes the administration of inhaled N2O or oral midazolam), 'someone other than the operator must be responsible for monitoring the patient'. If these agents are used together, the sedation technique is considered to be advanced, in which case 'someone other than the operator must be responsible for the administration of sedation, monitoring of vital signs and, should complications of sedation arise, rescue of the patient'.[4]
Deep sedation and general anaesthesia were less commonly administered than minimal and moderate sedation in this study. The distinction in depth is important, as the complication rate associated with planned mild to moderate sedation is lower than that with deep sedation (3.8% v. 9.2%; odds ratio 2.6).[2] The SASA PSA guideline[4] suggests that deep sedation and general anaesthesia should only be performed by those with anaesthetic training, in recognition of this increased risk. It is important to recognise, however, that the planes of sedation are difficult to predict, and that children can easily 'move' from moderate to deep sedation, with its attendant complications. For that reason, both a human 'monitor' and essential monitoring equipment are recommended, and any child receiving advanced sedation (i.e. more than one agent) should be fasted as per recommended guidelines for deep sedation or anaesthesia. It is recommended that all patients receiving PSA be monitored using pulse oximetry. Pulse oximetry, electrocardiography and non-invasive blood pressure monitoring are considered minimum monitoring for advanced sedation, with capnography recommended.[4] Of the practices surveyed in this report, 54.4% used pulse oximeters, but 41.3% did not use any form of monitoring equipment during PSA. This is in contrast to the 19% of North American practices that were found not to use pulse oximetry and the 5% that did not use any monitoring during PSA.[7]
Sedation should only be performed in an environment capable of handling emergencies.[4] While most dental practitioners in this study who provide PSA had Basic Life Support training, 43.2% of practices did not keep any of the recommended emergency or antidotal drugs in stock and 19.6% did not have any emergency equipment available.
Midazolam was the most frequently used sedative agent for paediatric dental chair PSA, while N2O was the most common agent administered in practices in which the dental practitioner was primarily responsible for PSA provision. Although N2O is generally considered to be safe, any agent can depress the patient's ability to respond normally to airway obstruction once the drug depresses the central nervous system.[8, 9]
Three or more sedative agents were combined in 28.1% of dental practices providing PSA. The potential for adverse outcomes is significantly increased when three or more drugs with sedative properties are combined.[3,4,10] This does not mean that drugs should not be combined, but emphasises the need for adequate monitoring and availability of emergency equipment when a combination of sedative agents is administered, as inadequate monitoring and equipment have been identified as being among the contributory causes of sedation-related adverse events.[4]
Drugs for PSA were most frequently given orally in this study (52.1%). There should nevertheless be a uniform level of vigilance, as any sedative has the potential to cause respiratory depression regardless of its route of administration.[10] Indeed, a review of paediatric dental sedation adverse effects from closed malpractice claims showed that 10 of 13 sedation claims involved administration of oral sedation.[11]
The majority of respondents (76.6%) in this study offered PSA to children <6 years of age. This is the age group most likely to require sedation to facilitate surgery, but also the group most vulnerable to the adverse effects associated with sedative medication.'11,121 Two dental practitioners indicated that they performed procedures under PSA in infants. An increased incidence of adverse sedation events in infants compared with other age groups has been reported,'131 and the SASA PSA guideline suggests that such patients should preferably be sedated in a hospital setting.[4]
Several dental practitioners gave further insights into issues they had encountered in the provision of PSA in the dental chair. Some are practical - people reported a limitation of both theatre time and the availability of anaesthetists to perform procedures in theatre. There are also financial considerations - participants commented on the reluctance of medical aid schemes to pay anaesthetists for dental chair sedation or to cover the extra costs associated with performing dental procedures in theatre. Despite this, some practitioners made use of the theatre setting as they were of the opinion that dental room sedation was 'too risky'.
It was highlighted that despite appreciating the risks associated with PSA, it is neither realistic nor necessary to restrict its provision to anaesthetists, who are insufficient in number to cater for the ever-increasing need for paediatric PSA.[9,14,15] Several practitioners indicated that they would like to offer PSA in their rooms, but felt that they first required training to be able to do so.
Conclusion
Performing dental procedures in children is often not possible without the aid of PSA, which if provided in dental rooms is cost-effective and overcomes the need to rely on the limited availability of anaesthetists. Patient safety should not, however, be compromised in any way.
Paediatric dental chair PSA was offered by 44.1% of dental practitioners interviewed in Gauteng. The modalities of PSA provided varied between dental practices, with many facilities not adhering to recommended safety standards. Particular areas of concern identified in this study were the high proportion of practices in which no monitoring equipment, emergency equipment or emergency drugs were available. More than 80% of the practitioners surveyed indicated an interest in attending sedation training, suggesting a desire to learn safe sedation techniques and comply with safety requirements. Increased emphasis on such training and promoting awareness of the PSA guideline may improve adherence to recommended safety standards.
We hope that this information will serve as a starting point to developing measures that will enhance safety during paediatric dental chair PSA in South Africa. We encourage the South African anaesthetic and dental communities to work together to achieve this.
Disclaimer. The views expressed in this article are those of the authors and not an official position of the University of the Witwatersrand. Source of support. Department of Anaesthesiology, University of the Witwatersrand.
Acknowledgement. This study fulfilled part of FB's course requirements for an MMed (Anaesthesiol) degree at the University of the Witwatersrand.
References
1. Roelofse JA. What's new in paediatric conscious sedation in dentistry? SAAD Dig 2010;26:3-7. [ Links ]
2. Hoffman GM, Nowakowski R, Troshynski TJ, Berens RJ, Weisman SJ. Risk reduction in pediatric procedural sedation by application of an American Academy of Pediatrics/American Society of Anesthesiologists process model. Pediatrics 2002;109(2):236-243. [http://dx.doi.org/10.1542/peds.109.2.236] [ Links ]
3. Cote CJ, Notterman DA, Karl HW, Weinberg JA, McCloskey C. Adverse sedation events in pediatrics: A critical incident analysis of contributing factors. Pediatrics 2000;105(4):805-814. [http://dx.doi.org/10.1542/peds.105.4.805] [ Links ]
4. South African Society of Anaesthesiologists. Guidelines for the safe use of procedural sedation and analgesia for diagnostic and therapeutic procedures in children. South Afr J Anaesth Analg 2010;16(5Suppl):S1-S37. [ Links ]
5. World Health Organization. WHO Patient Safety Research. France: World Health Organization, 2009 (WHO/IER/PSP/2009.10). [ Links ]
6. South African Dental Association. Find a practitioner. 2012. http://www.sada.co.za/d_nndpractitioner.asp. (accessed 23 June 2012). [ Links ]
7. Goodchild JH, Donaldson M. The use of sedation in the dental outpatient setting: A web-based survey of dentists. Dent Implantol Update 2011;22(11):73-80. [ Links ]
8. Vade A, Sukhani R, Dolenga M, Habisohn-Schuck C. Chloral hydrate sedation of children undergoing CT and MR imaging: Safety as judged by American Academy of Pediatrics guidelines. AJR Am J Roentgenol 1995;165(4):905-909. 'http://dx.doi.org/10.2214/ajr.165.4.76769901 [ Links ]
9. Cote CJ. Monitoring guidelines: Do they make a difference? AJR Am J Roentgenol 1995;165(4):910- 912. [http://dx.doi.org/10.2214/ajr.165.4.7676991] [ Links ]
10. Cote CJ, Karl HW, Notterman DA, Weinberg JA, McCloskey C. Adverse sedation events in pediatrics: Analysis of medications used for sedation. Pediatrics 2000;106(4):633-444. [http://dx.doi.org/10.1542/peds.106.4.633] [ Links ]
11. Chicka MC, Dembo JB, Mathu-Muju KR, Nash DA, Bush HM. Adverse events during pediatric dental anesthesia and sedation: A review of closed malpractice insurance claims. Pediatr Dent 2012;34(3):231-238. [ Links ]
12. Lee HH, Milgrom P, Starks H, Burke W. Trends in death associated with pediatric dental sedation and general anesthesia. Paediatr Anaesth 2013;23(8):741-746. [http://dx.doi.org/10.1111/pan.12210] [ Links ]
13. Malviya S, Voepel-Lewis T, Tait AR. Adverse events and risk factors associated with the sedation of children by nonanesthesiologists. Anesth Analg 1997;85(6):1207-1213. [http://dx.doi.org/10.1097/00000539-199712000-00005] [ Links ]
14. Krauss B, Green SM. Training and credentialing in procedural sedation and analgesia in children: Lessons from the United States model. Paediatr Anaesth 2008;18(1):30-35. [http://dx.doi.org/10.1111/j.1460-9592.2007.02406.x] [ Links ]
15. Smallman B. Pediatric sedation: Can it be safely performed by non-anesthesiologists? Curr Opin Anaesthesiol 2002;15(4):455-9. [http://dx.doi.org/10.1097/00001503-200208000-00008] [ Links ]
 Correspondence:
Correspondence:
F Bham
faizalbham@gmail.com
Accepted 21 April 2015.














