Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SAMJ: South African Medical Journal
versión On-line ISSN 2078-5135
versión impresa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.105 no.5 Pretoria may. 2015
http://dx.doi.org/10.7196/SAMJ.9649
CONTINUING MEDICAL EDUCATION
ARTICLE
Optimising the administration of antibiotics in critically ill patients
G A RichardsI; I A JoubertII; A J BrinkIII
IMB BCh, PhD, FCP (SA), FRCP; Division of Critical Care, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, and Charlotte Maxeke Johannesburg Academic Hospital, Johannesburg, South Africa
IIMB BCh, DA (SA), FCA (SA) (Critical Care); Division of Critical Care, Department of Anaesthesia, Faculty of Health Sciences, Groote Schuur Hospital and University of Cape Town, South Africa
IIIMB BCh, MMed (Clin Micro); Department of Clinical Microbiology, Ampath National Laboratory Services, Milpark Hospital, Johannesburg, South Africa
ABSTRACT
Optimal outcome and a reduction in the potential for resistance require that appropriate pharmacokinetic (PK) targets are achieved. Consequently, we need to target drug concentrations that are significantly higher than those conventionally presumed to be adequate.
Drug exposure varies according to the molecular weight, degree of ionisation, protein binding and lipid solubility of each agent. In critically ill patients, hypoalbuminaemia increases the free fraction of hydrophilic drugs, which in turn increases the volume of distribution and clearance (CL), both of which result in reduced drug levels. Similarly, augmented renal clearance (ARC), defined as a creatinine clearance (CLcr) of >130 mL/min/1.73 m2, which occurs frequently in critically ill patients, particularly younger patients with normal or near-normal creatinine levels, may also significantly reduce drug exposure. Studies have demonstrated a greater mortality and lower cure with ARC, particularly with the additive effects of obesity, hypoalbuminaemia and increasing resistance, if conventional dosages are used. These concepts apply to antibiotics targeting Gram-negative and -positive organisms. Knowledge of PK and the resistance profiles of organisms in each environment is necessary to prescribe appropriately. This article discusses these issues and the doses that should be used.
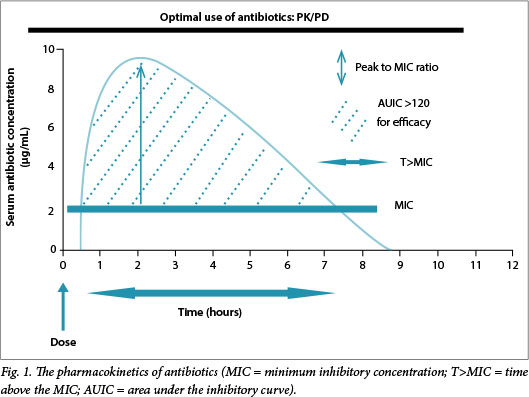
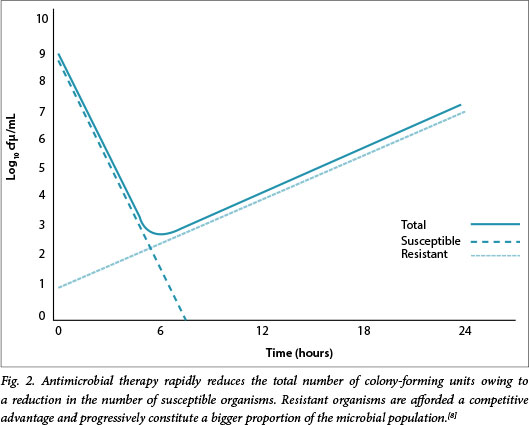
Optimal outcomes require achieving appropriate pharmacokinetic (PK) targets relative to the minimum inhibitory concentration (MIC) of the organism for a specific antibiotic. Antibiotics may be classified as time dependent, where a specific time above the MIC (T>MIC) is required to ensure optimal efficacy, and as concentration dependent, where the ratio of the area under the curve (AUC) to the MIC, also known as the area under the inhibitory curve (AUIC) or the peak-to-MIC ratio, more accurately reflects efficacy (Fig. 1). The AUIC might also be the most accurate parameter for some time-dependent drugs, particularly those with longer half-lives, such as the glycopeptides and linezolid. The optimal T>MIC for the β-lactams is >50% for penicillins, >60% for the cephalosporins and >40% for the carbapenems. For concentration-dependent agents the AUIC should generally be >120 or the peak-to-MIC ratio >8 - 10.[1,2] For example, in a study of free antibiotic levels, 248 patients with infection were examined to establish whether or not a target of 50% or 100% T>MIC was achieved. Those who did not achieve the 50% T>MIC target were significantly less likely to have a positive clinical outcome (odds ratio (OR) 0.68; p=0.009), and a positive clinical outcome was associated with increasing 50% T>MIC and 100% T>MIC ratios (OR 1.02 and 1.56, respectively; p<0.03).[3] Whereas these parameters primarily reflect efficacy, there is also the possibility that not achieving them may increase the potential for resistance, as selective pressure increases when there is a prolonged period below the MIC. Organisms that are more resistant have a lower AUIC and/or shorter T>MIC and an increased likelihood of survival.[4,5] Therefore, we might need to target drug concentrations that are significantly higher than those conventionally presumed to be adequate.[6] The mutant prevention concentration (MPC) is the concentration above which selective proliferation of mutants is unlikely to occur. Mutants are members of the microbial population with inherently higher MICs than the population average. Antibiotic concentrations that are targeted to the overall MIC would potentially be less than the MPC, thereby providing a competitive advantage to the mutant members of the microbial population.[7] Therefore, it is critical that the dose be optimised; some recommendations advise that concentrations should be >4 times the MIC for specific periods to prevent selection of resistant organisms - an essential component of antibiotic stewardship. Fig. 2 illustrates the concept of MPC and potential pitfalls of MIC-based dosing.[8]
Factors impacting on antibiotic exposure
Drug exposure varies according to molecular weight, degree of ionisation, protein binding and lipid solubility. Lipophilic antibiotics, e.g. the fluoroquinolones, have a large volume of distribution (Vd) owing to significant tissue and intracellular penetration. Hydrophilic agents, however, distribute into the extracellular space only and have a much lower Vd. The latter is influenced by a number of factors, such as serum albumin level, augmented renal clearance (ARC) and fluid losses as occurs with an open abdomen and major surgery with blood loss.[9]
Albumin level is of particular relevance for highly protein-bound antibiotics such as teicoplanin (90 - 95% bound), especially in critically ill patients in whom hypoalbuminaemia frequently occurs. In this setting, the Vd and clearance (CL) of the unbound/free fraction are increased.[10,11] These PK changes could result in suboptimal drug exposure, which may necessitate dose adjustments to ensure that therapeutic exposures are achieved.[12] In this regard, Mimoz et al.,[13] utilising a high-dose regimen of teicoplanin (12 mg/kg 12-hourly for 48 hours, followed by 12 mg/kg once daily) in critically ill patients with ventilator-associated pneumonia (VAP) and severe hypoalbuminaemia (median albumin concentration 16.1 g/L), observed variations in the fraction of unbound teicoplanin of 8 - 42%.
ARC is defined as a creatinine clearance (CLcr) >130 mL/min/1.73 m2. The prevalence varies from 30% to 85% in critically ill and trauma patients and a normal or near-normal creatinine level may represent a high glomerular filtration rate (GFR). At-risk populations are those with good physiological reserve, of a younger age and with lower illness severity scores. In this setting, dose increases are appropriate as the potential for subtherapeutic dosing is high. Increased β-lactam clearance in patients with sepsis, but without organ dysfunction, can lead to subtherapeutic levels for significant periods.[14-17] CLcr should be routinely measured if there is doubt about the GFR and evidence that an 8-hour collection may be just as accurate as a 24-hour one. A recent prospective, single-centre observational study of patients with VAP treated with doripenem or imipenem demonstrated a greater mortality and lower cure with CLcr >150 mL/min. Separate PK/pharmacodynamic (PD) modelling suggested that daily doripenem doses (up to 2 g 8-hourly) might be required for adequate drug exposure, particularly with resistant organisms.[17-19] In 128 surgical and medical patients encompassing 599 antibiotic days, ARC, defined as more than one 24-hour CLcr >130 mL/min/1.73m2, was present in 51.6% of patients and in 12% it occurred throughout the hospital stay. The median CLcr was 144 mL/min/1.73m2 (interquartile range (IQR) 98 - 196), the ARC patients were significantly younger (p<0.001) and treatment failure occurred more frequently: 27.3% v. 12.9%; p=0.04.[17] We investigated ertapenem PK in 8 patients with severe sepsis (all of whom had normal renal function) after the administration of the conventional dose of 1 g daily. These patients had a lower maximum concentration (Cmax), AUIC (0-∞), and higher Vd (26.8 L v. 5.7 L) than healthy volunteers, and in 4 patients time above 2 mg/L (the MIC breakpoint for Enterobacteriaceae) of the unbound fraction was <40% and in 2 it was <20%. These lower levels correlated negatively with low albumin, open abdomen and ARC.[20]
In summary, systemic inflammation increases the Vd of hydrophilic agents through capillary leak, large-volume crystalloid resuscitation and low albumin levels. Furthermore, altered organ per-fusion and therapeutic use of inotropes and vasopressors increase the potential for ARC. The additive effects of obesity and extracorporeal circuits reduce drug exposure in an environment where MICs are increasing inexorably. The overall effect is to increase the potential for treatment failure and select for resistance.[21]
What should be done to limit the impact of reduced drug exposure?
There are two obvious approaches, firstly to increase the dose and secondly to alter the methods of administration (infusion for time-dependent agents and, where possible, larger single daily doses for concentration-dependent drugs), both preferably guided by therapeutic drug monitoring (TDM).
β-lactams
In the abovementioned study by Claus et αl.,[17] doripenem was administered at four times the recommended dose - with good outcome. There have been many similar case studies of the outcomes when treating resistant organisms. In a patient with cystic fibrosis infected with multidrug-resistant Burkholderia cepacia, who was treated with meropenem 2 g 8-hourly as a 3-hour infusion, concentrations >8 μg/mL were achieved for 52% of the dosing interval, with subsequent improvement.[22] In a study of 348 patients using β-lactam therapy (the Defining Antibiotic Levels in ICU (DALI) study - a PK point prevalence study using empirical therapy in the 'worst case' scenario), T>MIC was <50% of the dosing interval in 19.2% and <100% in 41.4% of patients. Intermittent infusion significantly increased the likelihood of reaching the target, whereas increased CLcr was independently associated with not reaching the 100% T>MIC target for free drug.[23] Similarly, using a Monte Carlo simulation, Nicasio et al.[24] determined that 3-hour infusions of cefepime or meropenem, both at 2 g three times daily, would be most likely to achieve optimal bactericidal Pseudomonas aeruginosa exposure. When this was implemented, infection-related mortality decreased by 69% (8.5% v. 21.6%; p=0.029), length of stay was reduced (11.7±1 v. 26.1±18.5; p<0.001), there were fewer superinfections, and many 'non-susceptible' P. aeruginosa infections were successfully treated.
Tigecycline
The efficacy of tigecycline (TGC) has often been questioned. Meta-analyses of monotherapy v. comparators such as the meta-analysis by Yahav et al.[25] have been done. The latter included 15 trials (N=7 654) where overall mortality was higher (relative risk (RR) 1.29 (1.02 - 1.64)), regardless of infection type; clinical and microbiological failure were higher (RR 1.16 (1.06 - 1.27) and 1.13 (0.99 - 1.30), respectively); and development of septic shock was significantly more frequent (RR 7.01 (1.27 - 38.66)). However, numerous recent studies using increased doses have shown improved outcomes. Patients with hospital-acquired pneumonia were randomised to a 150 mg bolus and 75 mg 12-hourly or a 200 mg bolus and 100 mg 12-hourly v. imipenem 1 g 8-hourly.[26] Clinical cure with the larger dose (17/20; 85.0%) was numerically superior to that with the lower dose (16/23; 69.6%) and to imipenem (18/24; 75.0%). Despite the increased dose, there were no new safety signals. Their conclusion was that higher AUIC ratios may be necessary to achieve clinical cure in hospital-acquired pneumonia. Similarly, in a retrospective study of patients with VAP, in which the main isolates were carbapenem-resistant Acinetobacter (blaOXA-58 and blaOXA-23) and Klebsiella pneumoniae (blaKPC-3), high-dose TGC was compared with standard dose TGC.[27] Organisms were said to be TGC sensitive if the MIC <2 mg/L and resistant if the MIC was >8 mg/L. The single independent predictor of clinical cure was high-dose TGC (OR 6.25 (1.59 - 24.57); p=0.009).
Fluoroquinolones and aminoglycosides
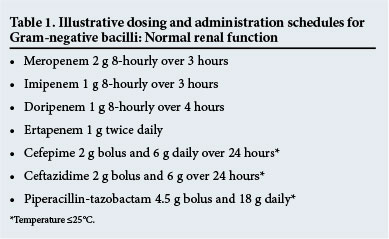
With regard to the concentration-dependent antibiotics, optimising the AUIC of fluoroquinolones reduced the development of resistance and was more likely to eradicate the pathogen.[28,29] Aminoglycosides are generally used suboptimally. To achieve appropriate targets, a much larger dose based on age and weight must be administered once per day and the MIC should be low.[30] In general, aminoglycosides are administered for short periods as empirical therapy to decrease the likelihood of inappropriate therapy for hospital-acquired infections. Peak and trough levels and the MIC of the organism (where possible) should be documented and subsequent doses titrated accordingly.[31] As with other hydrophilic agents where the Vd is increased and in the presence of ARC, concentrations may be suboptimal. Amikacin 15 mg/kg, for example, did not reach effective concentrations, with MICs of 8 mg/L, and it is possible that inconsistent concentrations may have contributed to the lack of effect in studies that investigated whether β-lactam-aminoglycoside combinations confer additional efficacy compared with β-lactams only.[32-35] Some reviewers have suggested that doses as high as 25 - 30 mg/kg for amikacin and 7 - 9 mg/kg for gentamicin or tobramycin should be administered initially, and thereafter a Cmax/MIC ratio of 8 - 10 should be targeted.[36] Even then, levels might not be adequate; 33% of patients receiving 25 mg/kg total body weight amikacin load had a Cmax of 60 mg/L, with positive fluid balance being the major negative predictive factor. To complicate matters further, Monte Carlo simulation of conventional v. high-dose extended-interval administration found resistance to be higher against pathogens with high MICs if T>MIC was <60%, even if Cmax/MIC was high, and that treatment efficacy may not be guaranteed.[37] Illustrative dosing schedules for Gram-negative agents may be seen in Table 1.
Colistin
Colistin is a last-line drug and if used inappropriately resistance will develop rapidly. The form available in South Africa is a prodrug, colistimethate sodium or colistin methanesulfonate (CMS), which makes a bolus dose necessary to achieve therapeutic effect. It is effective against most Gram-negative bacilli, except Proteus spp., B. cepacia, Providencia spp., Serratia marcescens and Morganella spp.[38] The appropriate dose must exceed an MIC of 2 mg/L rapidly to prevent regrowth of more resistant organisms in heteroresistant populations, in which the PK target achieved would be insufficient for eradication.[39]
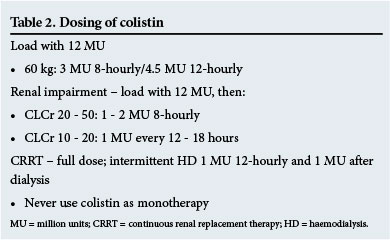
Consequently, a loading dose of12 million units (MU) administered intravenously over 1 - 2 hours followed by 9 MU daily (4.5 MU twice daily or 3 MU three times daily) administered 12 hours after the loading dose is required.[38,40,41] Colistin is predominantly cleared by unknown non-renal mechanisms and undergoes extensive renal tubular reabsorption.[42] In renal dysfunction, elimination of CMS is decreased and a greater fraction of the administered dose is converted to colistin; however, a loading dose of 12 MU is still required, but maintenance doses are reduced according to CLcr (Table 2).[40] From murine AUC/MIC colistin data, it is estimated that an AUIC of total colistin of 60 is the average achieved without exceeding the dose recommended in the package insert (10 MU), particularly where CLcr is >70 mL/min.[40] Therefore, in an attempt to reduce resistance, colistin is not administered as monotherapy and options include the carbapenems (provided the MIC is <32 mg/L (for carbapenem-resistant Enterobacteriaceae)), tigecycline (Acinetobacter), fluoroquinolones, rifampicin and others, even if the organism is resistant to these drugs.[43-45]
The glycopeptides, vancomycin and teicoplanin
These concepts regarding dosing are similar when using agents active against Gram-positive organisms. Vancomycin MICs have gradually been increasing, which appears to impact on outcome. In 158 patients with hospital-, ventilator- or healthcare-associated pneumonia caused by methicillin-resistant Staphylococcus aureus, 72.8% had vancomycin MIC >1.5 μg/mL. All-cause mortality at day 28 was 32.3%, but this increased as the MIC increased (p=0.001). Consequently, although controversial, it is recommended that other therapies be considered with MICs of 1 - 2 μg/mL.[46] Studies using higher troughs (15 - 20 mg/L), loading doses or continuous infusions differ with regard to improved clinical or microbiological outcome; however, it is hoped that higher dosing may delay resistance by not selecting those organisms with higher MICs.[47-50] In another study from the DALI group, 42 patients either received continuous infusions (CIs) (57%) or intermittent doses (43%) of vancomycin. The PK targets were a Cmin>15 mg/L or an AUIC >400 (assuming the MIC was 1 mg/L). The Cmin was highly variable and achieved in only 57% overall, and in 71% (CI) v. 39% (intermittent) (p=0.038). AUIC was achieved in 88% (CI) v. 50% (intermittent) (p=0.008). Whereas CI appeared to be superior, it was still not adequate in achieving targets, and multivariate analysis did not confirm CI as an independent predictor of either.[51]
Similarly, teicoplanin is unlikely to achieve therapeutic targets if administered in recommended doses as per the package insert. We performed a study in patients with normal renal function, in whom the standard dose of 400 mg twice daily x 1 day and 400 mg daily thereafter was compared with 400 mg twice daily.[52] With the latter, a Cmin of 15 mg/L was achieved only on day 3, whereas with the former it was never achieved. In another study of 10 patients with chronic bone sepsis, 800 mg twice daily was administered for 48 hours and then 800 mg daily. Samples were taken 15 minutes pre-, and 30 and 120 minutes post-teicoplanin dose. The CL of the free fraction (ff) was 33.5 L/hour (38.0 - 34.7) compared with the bound fraction 7.0 L/hour (6.8 - 9.8), and the major determinant of ff was albumin with an OR of 0.120 (0.078 - 0.161; p<0.001), with a lesser effect of total dose. This emphasises that multiple factors impact on serum levels whether or not the patients are critically ill.[53]
Linezolid
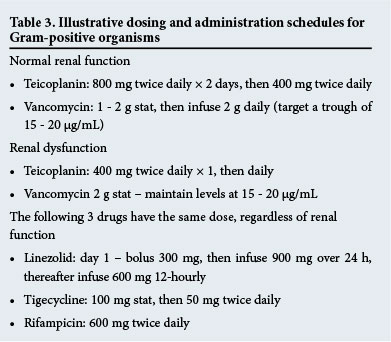
Linezolid is a time-dependent antibiotic and efficacy improves as the T>MIC increases. However, it is probably only with CI that this can this be achieved when MICs are higher.[54] Boselli et al.[55] demonstrated that a loading dose followed by CI led to concentrations twice that of a linezolid MIC of 4 mg/L in serum and epithelial lining fluid for 100% of the time in critically ill patients with VAP. Why is this important? A recent prospective observational study of 30 critically ill patients showed that levels were frequently inadequate with standard dosing of 600 mg twice daily. The range of the AUC24 was 50.1 - 453.9 mg*h/L (median 143.3) and that of Cmin 0.13 - 14.49 mg/L (median 2.06). Similarly AUC24 <200 mg*h/L and Cmin <2 mg/L, both of which represent inadequate levels, were observed for 63% and 50% of patients, respectively.[56] As the achievement of PK targets is essential, the dose and method of administration must be optimised, and it seems reasonable to utilise TDM where available; where not, CI might significantly improve AUIC.[57] This is not a review of TDM, but numerous other studies have proven its worth and it is probably the way of the future.[58] Illustrative dosing schedules for Gram-positive agents may be seen in Table 3.
Conclusion
There is currently a crisis with regard to antibiotic resistance. Every day that we delay ensures that we are further from a solution. We have to use antibiotics in an appropriate manner, reduce inappropriate use by all possible means, and reduce the incidence of infection, particularly in hospital. We are at the end of the antibiotic era -perhaps we can make it last a few more years to allow the introduction of new agents, particularly β-lactam antibiotics combined with β-lactamase inhibitors, or until new strategies can be devised.
References
1. Drusano GL. Prevention of resistance: A goal for dose selection for antimicrobial agents. Clin Infect Dis 2003;36(Suppl 1):S42-S50. [ Links ]
2. Craig WA. Antimicrobial resistance issues of the future. Diagn Microbiol Infect Dis 1996;25(4):213-217. [ Links ]
3. Roberts JA, Paul SK, Akova M, et al., DALI Study. DALI: Defining antibiotic levels in intensive care unit patients: Are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis 2014;58:1072-1083. [http://dx.doi.org/10.1093/cid/ciu027] [ Links ]
4. Baquero F, Negri MC, Morosini MI, Blazquez J. Antibiotic-selective environments. Clin Infect Dis 1998;27(Suppl 1):S5-S11. [ Links ]
5. Baquero F. Low-level antibacterial resistance: A gateway to clinical resistance. Drug Resist Updat 2001;4(2):93-105. [ Links ]
6. Oloffson S, Cars O. Optimizing drug exposure to minimize selection of antibiotic resistance. Clin Infect Dis 2007;45(Suppl):S129-S136. [ Links ]
7. Sanders CC, Sanders WE. Type I β-lactamases of gram-negative bacteria: Interactions with β-lactam antibiotics. J Infect Dis 1986;154:792-800. [ Links ]
8. Drusano GL. Antimicrobial pharmacodynamics: Critical interactions of 'bug and drug. Nat Rev Microbiol 2004;2:289-300. [ Links ]
9. Udy AA, Roberts JA, Lipman J. Clinical implications of antibiotic pharmacokinetic principles in the critically ill. Intensive Care Med 2013;39:2070-2082. [http://dx.doi.org/10.1007/s00134-013-3088-4] [ Links ]
10. Ulldemolins M, Roberts JA, Rello J, et al. The effects of hypoalbuminaemia on optimizing antibiotic dosing in critically ill patients. Clin Pharmacokinet 2011;50:1-12. [http://dx.doi.org/10.2165/11539220-000000000-00000] [ Links ]
11. Vincent JL, Russell JA, Jacob M, et al. Albumin administration in the acutely ill: What is new and where next? Crit Care 2014;18:231. [http://dx.doi.org/10.1186/cc13991] [ Links ]
12. Roberts JA, Pea F, Lipman J. The clinical relevance ofplasma protein binding changes. Clin Pharmacokinet 2013;52:1-8. [http://dx.doi.org/10.1007/s40262-012-0018-5] [ Links ]
13. Mimoz O, Rolland D, Adoun M, et al. Steady-state trough serum and epithelial lining fluid concentrations of teicoplanin 12 mg/kg per day in patients with ventilator-associated pneumonia. Intensive Care Med 2006;32:775-779. [ Links ]
14. Minville V, Asehnoune K, Ruiz S, et al. Increased creatinine clearance in polytrauma patients with normal serum creatinine: A retrospective observational study. Crit Care 2011;15:R49. [http://dx.doi.org/10.1186/cc10013] [ Links ]
15. Fuster-Lluch O, Geronimo-Pardo M, Peyro-Garcia R, Lizan-Garcia M. Glomerular hyperfiltration and albuminuria in critically ill patients. Anaesth Intensive Care 2008;36(5):674-680. [ Links ]
16. Udy AA, Roberts JA, Shorr AF, Boots RJ, Lipman J. Augmented renal clearance in septic and traumatized patients with normal plasma creatinine concentrations: Identifying at-risk patients. Crit Care 2013;17(1):R35. [http://dx.doi.org/10.1186/cc12544] [ Links ]
17. Claus BO, Hoste EA, Colpaert K, Robays H, Decruyenaere J, De Waele JJ. Augmented renal clearance is a common finding with worse clinical outcome in critically ill patients receiving antimicrobial therapy. J Crit Care 2013;28(5):695-700. [http://dx.doi.org/10.1016/j.jcrc.2013.03.003] [ Links ]
18. Kollef MH, Chastre J, Clavel M, et al. A randomized trial of 7-day doripenem versus 10-day imipenem-cilastatin for ventilator-associated pneumonia. Crit Care 2012;16(6):R218. [http://dx.doi.org/10.1186/cc11862] [ Links ]
19. Roberts JA, Lipman J. Optimal doripenem dosing simulations in critically ill nosocomial pneumonia patients with obesity, augmented renal clearance, and decreased bacterial susceptibility. Crit Care Med 2013;41:489-495. [http://dx.doi.org/10.1097/CCM.0b013e31826ab4c4] [ Links ]
20. Brink AJ, Richards GA, Schillack V, Kiem S, Schentag J. Pharmacokinetics of once-daily dosing of ertapenem in critically ill patients with severe sepsis. Int J Antimicrob Agents 2009;33:432-436. [http://dx.doi.org/10.1016/j.ijantimicag.2008.10.005] [ Links ]
21. Udy AA, Roberts JA, Boots RJ, Paterson DL, Lipman J. Augmented renal clearance: Implications for antibacterial dosing in the critically ill. Clin Pharmacokinet 2010; 49(1):1-16. [http://dx.doi.org/10.2165/11318140-000000000-00000] [ Links ]
22. Kuti JL, Moss KM, Nicolau DP, Knauft RF. Empiric treatment of multidrug-resistant Burkholderia cepacia lung exacerbation in a patient with cystic fibrosis: Application of pharmacodynamic concepts to meropenem therapy. Pharmacotherapy 2004;24:1641-1645. [ Links ]
23. De Waele JJ, Lipman J, Akova M, et al. Risk factors for target non-attainment during empirical treatment with β-lactam antibiotics in critically ill patients. Intensive Care Med 2014;40:1340-1351. [http://dx.doi.org/10.1007/s00134-014-3403-8] [ Links ]
24. Nicasio AM, Eagye KJ, Nicolau DP, et al. Pharmacodynamic-based clinical pathway for empiric antibiotic choice in patients with ventilator-associated pneumonia. J Crit Care 2010;25(1):69-77. [http://dx.doi.org/10.1016/j.jcrc.2009.02.014] [ Links ]
25. Yahav D, Lador A, Paul M, Leibovici L. Efficacy and safety of tigecycline: A systematic review and meta-analysis. J Antimicrob Chemother 2011;66:1963-1971. [http://dx.doi.org/10.1093/jac/dkr242] [ Links ]
26. Ramirez J, Dartois N, Gandjini H, Yan JL, Korth-Bradley J, McGovern PC. Randomized phase 2 trial to evaluate the clinical efficacy of two high-dosage tigecycline regimens versus imipenem-cilastatin for treatment of hospital-acquired pneumonia. Antimicrob Agents Chemother 2013;57:1756-1762. [http://dx.doi.org/10.1128/AAC.01232-12] [ Links ]
27. De Pascale G, Montini L, Pennisi M, et al. High dose tigecycline in critically ill patients with severe infections due to multidrug-resistant bacteria. Crit Care 2014;18:R90. [http://dx.doi.org/10.1186/cc13858] [ Links ]
28. Thomas JK, Forrest A, Bhavnani SM, et al. Pharmacodynamic evaluation of factors associated with the development of bacterial resistance in acutely ill patients during therapy. Antimicrob Agents Chemother 1998;42:521-527. [ Links ]
29. Forrest A, Nix DE, Ballow CH, Goss TF, Birmingham MC, Schentag JJ. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother 1993;37:1073-1081. [ Links ]
30. Turnidge J. Pharmacodynamics and dosing of aminoglycosides. Infect Dis Clin North Am 2003;17:503-528. [ Links ]
31. Kumar A, Kethireddy S. Emerging concepts in optimizing antimicrobial therapy of septic shock: Speed is life but a hammer helps too. Crit Care 2013;17(1):104. [http://dx.doi.org/10.1186/cc11890] [ Links ]
32. Paul M, Lador A, Grozinsky-Glasberg S, Leibovici L. Beta lactam antibiotic monotherapy versus beta lactam-aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Syst Rev 2014;7(1):CD003344. [http://dx.doi.org/10.1002/14651858.CD003344.pub3] [ Links ]
33. Bliziotis IA, Petrosillo N, Michalopoulos A, Samonis G, Falagas ME. Impact of definitive therapy with beta-lactam monotherapy or combination with an aminoglycoside or a quinolone for Pseudomonas aeruginosa bacteremia. PLoS One 2011;6(10):e26470. [http://dx.doi.org/10.1371/journal.pone.0026470] [ Links ]
34. Taccone FS, Laterre PF, Spapen H, et al. Revisiting the loading dose of amikacin for patients with severe sepsis and septic shock. Crit Care 2010;14(2):R53. [http://dx.doi.org/10.1186/cc8945] [ Links ]
35. Tamma PD, Cosgrove SE, Maragakis LL. Combination therapy for treatment of infections with gram-negative bacteria. Clin Microbiol Rev 2012;25:450-470. [http://dx.doi.org/10.1128/CMR.05041-11] [ Links ]
36. Matthaiou DK, De Waele J, Dimopoulos G. What is new in the use of aminoglycosides in critically ill patients? Intensive Care Med 2014;40(10):1553-1555. [http://dx.doi.org/10.1007/s00134-014-3376-7] [ Links ]
37. Zazo H, Martin-Suarez A, Lanao JM. Evaluating amikacin dosage regimens in intensive care unit patients: A pharmacokinetic/pharmacodynamic analysis using Monte Carlo simulation. Int J Antimicrob Agents 2013;42:155-160. [http://dx.doi.org/10.1016/j.ijantimicag.2013.04.021] [ Links ]
38. Plachouras D, Karvanen M, Friberg LE, et al. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by Gram-negative bacteria. Antimicrob Agents Chemother 2009;53(8):3430-3436. [http://dx.doi.org/10.1128/AAC.01361-08] [ Links ]
39. Chun-Hong T, Jian Li, Roger L. Activity of colistin against heteroresistant Acinetobacter baumannii and emergence of resistance in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother 2007;51:3413-3415. [http://dx.doi.org/10.1128/AAC.01571-06] [ Links ]
40. Garonzik SM, Li J, Thamlikitkul V, et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 2011;55:3284-3294. [http://dx.doi.org/10.1128/AAC.01733-10] [ Links ]
41. Kift EV, Maartens G, Bamford C. Systematic review of the evidence for rational dosing of colistin. S Afr Med J 2014;104:183-186. [ Links ]
42. Li J, Milne RW, Nation RL, Turnidge JD, Smeaton TC, Coulthard K. Use of high-performance liquid chromatography to study the pharmacokinetics of colistin sulfate in rats following intravenous administration. Antimicrob Agents Chemother 2003;47(5):1766-1770. [ Links ]
43. Dalfino L, Puntillo F, Mosca A, et al. High-dose, extended-interval colistin administration in critically ill patients: Is this the right dosing strategy? A preliminary study. Clin Infect Dis 2012;54:1720-1726. [http://dx.doi.org/10.1093/cid/cis28640] [ Links ]
44. Petrosillo N, Giannella M, Lewis R, Viale P. Treatment of carbapenem-resistant Klebsiella pneumoniae: The state of the art. Expert Rev Anti Infect Ther 2013;11:159-177. [http://dx.doi.org/10.1586/eri.12.162] [ Links ]
45. Tumbarello M, Viale P, Viscoli C, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: Importance of combination therapy. Clin Infect Dis 2012;55:943-950. [http://dx.doi.org/10.1093/cid/cis588] [ Links ]
46. Haque NZ, Zuniga LC, Peyrani P, et al.; Improving Medicine through Pathway Assessment of Critical Therapy of Hospital-Acquired Pneumonia (IMPACT-HAP) Investigators. Relationship of vancomycin minimum inhibitory concentration to mortality in patients with methicillin-resistant Staphylococcus aureus hospital-acquired, ventilator-associated, or health-care-associated pneumonia. Chest 2010;138:1356-1362. [http://dx.doi.org/10.1378/chest.09-2453] [ Links ]
47. Brink AJ. Does resistance in severe infections caused by methicillin-resistant Staphylococcus aureus give you the 'creeps'? Curr Opin Crit Care 2012;18:451-459. [http://dx.doi.org/10.1097/MCC.0b013e3283578968] [ Links ]
48. Van Hal SJ, Paterson DL. Systematic review and meta-analysis of the significance of heterogeneous vancomycin-intermediate Staphylococcus aureus isolates. Antimicrob Agents Chemother 2011;55:405-410. [http://dx.doi.org/10.1128/AAC.01133-10] [ Links ]
49. Dhand A, Sakoulas G. Reduced vancomycin susceptibility among clinical Staphylococcus aureus isolates ('the MIC Creep'): Implications for therapy. F1000 Med Rep 2012;4:4. [http://dx.doi.org/10.3410/M4-4] [ Links ]
50. Nannini E, Murray BE, Arias CA. Resistance or decreased susceptibility to glycopeptides, daptomycin, and linezolid in methicillin-resistant Staphylococcus aureus. Curr Opin Pharmacol 2010;10:516-521. [http://dx.doi.org/10.1016/j.coph.2010.06.006] [ Links ]
51. Blot S, Koulenti D, Akova M, et al. Does contemporary vancomycin dosing achieve therapeutic targets in a heterogeneous clinical cohort of critically ill patients? Data from the multinational DALI study. Crit Care 2014;18:R99. [http://dx.doi.org/10.1186/cc13874] [ Links ]
52. Brink AJ, Richards GA, Cummins RR, Lambson J; Gauteng Understanding Teicoplanin Serum levels (GUTS) study group. Recommendations to achieve rapid therapeutic teicoplanin plasma concentrations in adult hospitalised patients treated for sepsis. Int J Antimicrob Agents 2008;32:455-458. [http://dx.doi.org/10.1016/j.ijantimicag.2008.05.012] [ Links ]
53. Brink AJ, Richards GA, Lautenbach C, et al. A post-authorisation survey to analyse the perioperative teicoplanin plasma concentrations in adult patients with chronic bone sepsis, who received loading doses of 12 mg/kg 12-hourly for 48 hours followed by 12 mg/kg once daily. Crit Care 2012;16(1):3. [http://dx.doi.org/10.1186/cc10676] [ Links ]
54. Adembri C, Fallani S, Cassetta MI, et al. Linezolid pharmacokinetic/pharmacodynamic profile in critically ill septic patients: Intermittent versus continuous infusion. Int J Antimicrob Agents 2008;31:122-129. [ Links ]
55. Boselli E, Breilh D, Caillault-Sergent A, et al. Alveolar diffusion and pharmacokinetics of linezolid administered in continuous infusion to critically ill patients with ventilator-associated pneumonia. Antimicrob Chemother 2012;67:1207-1210. [ Links ]
56. Zoller M, Maier B, Hornuss C, et al Variability of linezolid concentrations after standard dosing in critically ill patients: A prospective observational study. Crit Care 2014;10;18:R148. [http://dx.doi.org/10.1186/cc13984] [ Links ]
57. Richards GA, Brink AJ. Therapeutic drug monitoring: Linezolid too? Crit Care 2014;18:525-526. [ Links ]
58. De Waele JJ, Carrette S, Carlier M, et al. Therapeutic drug monitoring-based dose optimisation of piperacillin and meropenem: A randomised controlled trial. Intensive Care Med 2014;40:380-387. [http://dx.doi.org/10.1007/s00134-013-3187-2] [ Links ]
 Correspondence:
Correspondence:
G A Richards
guy.richards@wits.ac.za














