Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.105 no.5 Pretoria Mai. 2015
http://dx.doi.org/10.7196/SAMJ.9665
EDITORIAL
Antibiotic administration in the critically ill - in need of intensive care!
Infections and infectious diseases remain a leading cause of morbidity and mortality worldwide. Sepsis claims 10 000 lives globally every day.[1] Antimicrobials, a major weapon in our armamentarium to combat infections, are arguably the most poorly prescribed of all medications. Antibiotics represent at least 30% of acute care hospitals' drug expenditure. They are prescribed to 20 - 50% of inpatients and to a greater extent in intensive care unit (ICU) patients. Of note they are prone to misuse, with 22 - 65% of prescriptions either not indicated or inadequate to treat the infec-tion.[2,3,4] The consequences of this misuse are unnecessary costs and side-effects, resistant micro-organisms and failure of treatment.[2,5,6] The Prevalence of Infection in South Africa (PISA) study, which evaluated antimicrobial prescription practices at specialist and super-specialist level in state/academic and the private sectors among critically ill patients in South Africa (SA), revealed startling results -approximately 80% of such patients were receiving antimicrobial therapy, the appropriate choice of antimicrobial occurred in just over 40% of patients, antibiotics were modified appropriately in just over 10% of patients, and the duration of therapy was appropriate in just over a quarter of the patients.[3]
A recent commentary aptly suggested that the major reason that antibiotics are prescribed inappropriately is that there is a lack of knowledge about infectious diseases and antimicrobial therapy, and health care providers are afraid not to prescribe antibiotics.[7] This is particularly true in the critical care setting, where antimicrobial management represents an ongoing challenge.
Critically ill patients constitute a unique population and differ from the non-critically ill in terms of antibiotic administration and dosing. A greater understanding of antibiotic dosing in these patients is essential for all involved in their care. This editorial aims to focus on some of the important principles and strategies aimed at optimising antimicrobial use among critically ill patients.
In a recent study measuring serum β-lactam antibiotic concentrations in patients in the ICU, it was found that almost three-quarters of antibiotic prescriptions needed to be altered to achieve therapeutic targets (plasma concentrations too low) without toxicity (plasma concentrations too high).[8]
The reason for the inaccuracy of a 'one size fits all' dosing in the ICU, apart from patients' weights, involves the distinct patho-physiological changes that occur in ICU patients and their management. The main alterations in this regard relating to antibiotic concentrations are: (i) increased volume of distribution of drugs; (ii) increased cardiac output; (iii) increased hepatic and renal blood flow (and hence increased metabolism and excretion); and (iv) low serum protein levels, and hence altered protein binding of drugs (Fig. 1).[9,10] These changes often lead to subtherapeutic concentrations. This may be further compounded by the fact that ICU patients frequently have renal dysfunction and require renal replacement therapies (RRTs), which further complicates the resultant serum antibiotic concentration(s). Depending on the dose prescribed of both drug and RRT, there could potentially be under- or over-dosing.[8]

Patients with sepsis tend to need, and to be given, fluid in the initial resuscitative phase of the disease. Leaky capillaries, often compounded by hypoproteinaemia, predispose these patients to extravascular fluid extravasation. This will have little effect on lipophilic agents (e.g. fluoroquinolones) as their typical volume of distribution (Vd; the space into which the drug diffuses) is very large, and the relative increase in Vd too small to produce an overall Vd change.[11] Hydrophilic antibiotics, which primarily occupy the intravascular space, will also distribute into this increased extravascular water, and due to a relatively small initial Vd, this increase will produce a markedly large change (increase) in the Vd of such antibiotics. This increased Vd means that administering the same dose of a hydrophilic antibiotic to a patient with leaky capillaries will result in a lower concentration of the antibiotic in the serum, and particularly a lower maximal concentration. Marik[12] demonstrated that the sicker the patient (and the higher the APACHE II score), the larger the Vd of amikacin. This phenomenon (i.e. increased Vd, needing larger doses for the same serum concentration in critically ill patients) has been validated by others.
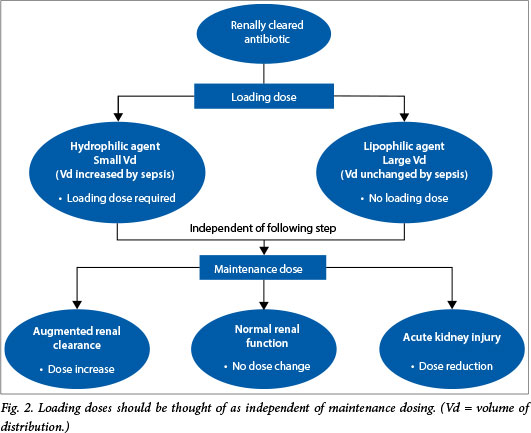
Practical implications for dosing. As first dose give a large, loading dose of antibiotic, particularly those with hydrophilic tendencies (aminoglycosides, glycopeptides, β-lactams and colistin.) Remember this is independent of altered clearances (i.e. renal dysfunction) (Fig. 2).
Di Giantomasso et al. [13] have demonstrated an increased organ blood flow early in sepsis. Clinically this means that in the presence of normal renal function, an increased renal blood flow will translate into an increased glomerular filtration rate and hence an increased creatinine clearance. This clinical phenomenon has now been termed augmented renal clearance (ARC).[14] In such circumstances all renally eliminated drugs will have increased clearances. ARC has now been documented in >60% of patients with 'normal' serum creatinine admitted to multidisciplinary ICUs.[15] The practical implication is that with standard dosing, ARC results in subtherapeutic concentrations of drugs that have renal elimination (unless higher doses are administered) (Fig. 1).
Practical implications for dosing. Younger patients without renal dysfunction often manifest ARC and hence clear renally eliminated drugs quickly. Shorten the dosing interval, e.g. instead of daily aminoglycosides use 18-hourly; instead of a bi-daily dose of β-lactam administer same dose 8-hourly. Increased total daily dose (aminoglycosides, glycopeptides, β-lactams), extended infusions (β-lactams) and therapeutic drug monitoring (TDM) may also be employed.
Highly protein-bound drugs are usually kept within the vascular compartment owing to the size of the protein-drug complex. In such circumstances, and depending on a number of other factors, there is usually a small amount of circulating drug that is not protein bound. This component (free drug, f) represents the active constituent of the drug and crosses various membranes such as vascular, kidney and meninges. Protein binding of <70% is usually of little clinical consequence, but commonly used antibiotics such as ertapenem, teicoplanin, ceftriaxone, flucloxacillin and daptomycin are highly protein bound (>80% protein binding).
In a pragmatic trial of 7 000 adult patients requiring fluid resuscitation who were admitted to ICUs in Australia and New Zealand, 40% of patients arrived in the ICU with albumin concentrations of <25 g/L.[16] As albumin is the primary binding site for most drugs, if hypoalbuminaemic patients are administered highly protein-bound drugs, there will initially be a much higher than normal f. If the drug is renally excreted and the patient has normal or near normal renal function, this high free fraction will soon pass through the kidney (via glomerular filtration) and be eliminated from the body at a rate much higher than occurs with the 'normal' high protein binding.[17] In such circumstances the half-life of these drugs is much shorter than expected. Clinically this has the effect of shortening the duration of action of these drugs. ARC further exaggerates this effect in that the antibiotic half-life and duration of action are even shorter (Fig. 1).
Practical implications for dosing. For highly protein-bound drugs in the ICU, shorten the frequency of dosing, e.g. instead of daily ceftriaxone administer a bi-daily dose; similarly for ertapenem, teicoplanin and daptomycin, higher than 'standard' doses are suggested. Larger loading doses (aminoglycosides, glycopeptides, β-lactams), extended infusions (β-lactams) and TDM may also be employed.
For renally eliminated antibiotics there are reasonably good texts for dosage adjustments in patients with renal dysfunction, and to a lesser extent this is also the case with hepatic dysfunction. RRT alters clearances markedly, and this complicates dosage administration enormously.[18-23] ICUs order their RRT modalities differently (CVVH, CVVHD, CVVHDF, SLEDD) and even the settings within these modalities differ (predilution, post-dilution, dialysate rates). Clearances of urea, creatinine, fluids and in fact medium and small molecules all vary in these different modalities. It is therefore not surprising that clearances of the unbound component of antibiotics will differ accordingly. In the clinical scenario a prescription of continuous RRT does not necessarily imply a 24-hour clearance time, as there is often 'downtime' of such artificial kidneys relating to entities such as kidney clotting and set-up times for new circuits. This complicates the clinical clearances even further (Fig. 1). It is for this reason that some units routinely employ TDM to prescribe antibiotics more accurately, including β-lactam TDM.[1,24,25,26]
This is standard practice, for example, at the Royal Brisbane and Women's Hospital.
Practical implications for dosing. Search for an article measuring antibiotic concentrations with the RRT settings most similar to your own and use this article to adjust your dosing, or ask your laboratory to set up β-lactam TDM. Note too that if continuous RRT filter downtime is prolonged (nurses busy, long set-up time) clearances will be far less than continuous 24-hour continuous RRT.
There is a global problem of increasing antibiotic resistance, with the minimum inhibitory concentration (MIC) of many organisms rising. While it is beyond the scope of this editorial to address single or double Gram-negative antibiotic cover, or how to deal with increasing resistance patterns optimally, it is important to recognise that increasing the dose of some antibiotics can overcome rising MICs.
How to deliver β-lactam antibiotics optimally has received much attention in recent literature (i.e. via a bolus or prolonged infusions, be they continuous infusion or extended infusions). There is some emerging evidence that delivery of β-lactam antibiotics may be further enhanced by administration as a prolonged infusion.[27,28] Where continuous infusions are employed, an initial bolus dose should be given prior to the initiation of the infusion. It should be noted that, to date, continuous infusions have not been shown to improve clinical outcomes. Our personal advice is to use extended infusions.
Finally, we would like to conclude by addressing the issue of β-lactam TDM. TDM in general can be used to prevent toxicity or improve efficacy. Measurement of aminoglycoside and vancomycin serum concentrations, which are generally universally available, usually fall into the former category. As β-lactams have a high therapeutic range with infrequent toxicity, it was conventionally deemed that TDM of these drugs was unnecessary. More recently we have realised how we have been underdosing such drugs, and the need for TDM of these antibiotics has come to the fore.[1,24,25,26]
A better understanding of antibiotic dosing in the critically ill will go a long way to enhancing the longevity of what is becoming an increasingly scarce resource. There are no new antibiotic classes nearing clinical production. We believe that correct antibiotic dosing will limit the increasing burden of antimicrobial resistance, minimise therapeutic failures and, most importantly, improve patient outcomes.
M Mer
Divisions of Critical Care and Pulmonology, Department of Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
J Lipman
Director, Department of Intensive Care Medicine, Royal Brisbane and Women's Hospital, Brisbane, Australia, and Professor and Head of Anaesthesiology and Critical Care, University of Queensland, Queensland, Australia
References
1. Global Sepsis Alliance. Stop Sepsis. Save Lives. www.globalsepsisalliance.org (accessed 11 January 2015). [ Links ]
2. Von Gunten V, Reymond JP, Boubaker K, et al. Antibiotic use: Is appropriateness expensive? J Hosp Infect 2009;71(2):108-111. [http://dx.doi.org/10.1016/j.jhin.2008.10.026] [ Links ]
3. Paruk F, Richards G, Scribante J, et al. Antibiotic prescription practices and their relationship to outcome in South Africa: Findings of the prevalence of infection in South African intensive care units (PISA) study. S Afr Med J 2012;102(7):613-616 [ Links ]
4. Roberts JA, Paul SK, Akova M, et al. DALI: Defining antibiotic levels in intensive care unit patients: Are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis 2014;58(8):1072-1083. [http://dx.doi.org/10.1093/cid/ciu027] [ Links ]
5. Dellit TH, Owens RC, McGowan JE, et al. Infectious Disease Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 2007;44(2):159-177. [http://dx.doi.org/10.1086/510393] [ Links ]
6. Paterson DL, Lipman J. Returning to the pre-antibiottic era in the critically ill: The XDR problem. Crit Care Med 2007;35(7):1789-1791. [http://dx.doi.org/10.1097/01.CCM.0000269352.39174.A4] [ Links ]
7. Nouwen JL. Controlling antibiotic use and resistance. Clin Infect Dis 2006; 42(6):776-777. [http://dx.doi.org/10.1086/500328] [ Links ]
8. Roberts JA, Ulldemolins M, Roberts MS, et al. Therapeutic drug monitoring of β-lactams in critically ill patients: Proof of concept. Int J Antimicrob Agents 2010;36(4):332-339. [http://dx.doi.org/10.1016/j.ijantimicag.2010.06.008] [ Links ]
9. Udy AA, Roberts JA, De Waele JJ, Paterson DL, Lipman J. What's behind the failure of emerging antibiotics in the critically ill? Understanding the impact of altered pharmacokinetics and augmented renal clearance. Int J Antimicrob Agents 2012;39(6):455-457. [http://dx.doi.org/10.1016/j.ijantimicag.2012.02.010] [ Links ]
10. Udy AA, Roberts JA, Lipman J. Clinical implications of antibiotic pharmacokinetic principles in the critically ill. Intensive Care Med 2013;39(12):2070-2082. [http://dx.doi.org/10.1007/s00134-013-3088-4] [ Links ]
11. Blot SI, Pea F, Lipman J. The eflffect of pathophysiology on pharmacokinetics in the critically ill patient - concepts appraised by the example of antimicrobial agents. Adv Drug Deliver Rev 2014;77:3-11. [http://dx.doi.org/10.1016/j.addr.2014.07.006] [ Links ]
12. Marik PE. Aminoglycoside volume of distribution and illness severity in critically ill septic patients. Anaesth Intensive Care 1993;21(2):172-173. [ Links ]
13. Di Giantomasso D, May CN, Bellomo R. Vital organ blood flow during hyperdynamic sepsis. Chest 2003;124(3):1053-1059. [http://dx.doi.org/10.1378/chest.124.3.1053] [ Links ]
14. Udy AA, Roberts JA, Boots RJ, Paterson DL, Lipman J. Augmented renal clearance. Clin Pharmacokinet 2010;49(1):1-16. [http://dx.doi.org/10.2165/11318140-000000000-00000] [ Links ]
15. Udy AA, Baptista JP, Lim NL, et al. Augmented renal clearance in the ICU: Results of a multicenter observational study of renal function in critically ill patients with normal plasma creatinine concentrations. Crit Care Med 2014;42(3):520-527. [http://dx.doi.org/10.1097/CCM.0000000000000029] [ Links ]
16. Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R; SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 2004;350:2247-2256. [http://dx.doi.org/10.1056/NEJMoa040232] [ Links ]
17. Joynt GM, Lipman J, Gomersall CD, Young RJ, Wong EL, Gin T. The pharmacokinetics of once-daily dosing of ceftriaxone in critically ill patients. J Antimicrob Chemother 2001;47(4):421-429. [htttp://dx.doi.org/10.1093/jac/47.4.421] [ Links ]
18. Eyler RF, Mueller BA. Antibiotic dosing in critically ill patients with acute kidney injury. Nat Rev Nephrol 2011;7(4):226-235. [http://dx.doi.org/10.1038/nrneph.2011.12] [ Links ]
19. Kielstein JT, Burkhardt O. Dosing of antibiotics in critically ill patients undergoing renal replacement therapy. Curr Pharm Biotechnol 2011;12(12):2015-2019. [http://dx.doi.org/10.2174/138920111798808275] [ Links ]
20. Roberts DM, Roberts JA, Roberts MS, et al. Variability of antibiotic concentrations in critically ill patients receiving continuous renal replacement therapy: A multicentre pharmacokinetic study. Crit Care Med 2012;40(5):1523-1528. [http://dx.doi.org/10.1097/CCM.0b013e318241e553] [ Links ]
21. Blot S, Lipman J, Roberts DM, et al. The influence of acute kidney injury on antimicrobial dosing in critically ill patients: Are dose reductions always necessary? Diagn Microbiol Infect Dis 2014:79(1):77-84. [http://dx.doi.org/10.1016/j.diagmicrobio.2014.01.015] [ Links ]
22. Roberts JA, Roberts DM. Antibiotic dosing in critically ill patients with septic shock and on continuous renal replacement therapy: Can we resolve this problem with pharmacokinetic studies and dosing guidelines? Crit Care 2014;18(3):156. [http://dx.doi.org/10.1186/cc13939] [ Links ]
23. Kumar A, Singh NP. Antimicrobial dosing in critically ill patients with sepsis-induced acute kidney injury. Indian J Crit Care Med 2015;19(2):99-108. [http://dx.doi.org/10.4103/0972-5229.151018] [ Links ]
24. McWhinney B, Wallis S, Hillister T, Roberts JA, Lipman J, Ungerer JPJ. Analysis of twelve beta-lactam antibiotics in human plasma by HPLC with ultraviolet detection. J Chromatogr B 2010;878(22):2039-2043. [http://dx.doi.org/10.1016/j.jchromb.2010.05.027] [ Links ]
25. Udy AA, De Waele JJ, Lipman J. Augmented renal clearance and therapeutic monitoring of β-lactams. Int J Antimicrob Agents 2015;45(4):331-333. [http://dx.doi.org/10.1016/j.ijantimicag.2014.12.020] [ Links ]
26. Wong G, Brinkman A, Benefield RJ, et al. An international, multi-centre survey of β-lactam antibiotics therapeutic drug monitoring practice in intensive care units. J Antimicrob Chemother 2014;69(5):1416-1423.[http://dx.doi.org/10.1093/jac/dkt523] [ Links ]
27. Falagas ME, Tansarli GS, Ikawa K, Vardakas KZ. Clinical outcomes with extended or continuous versus short-term intravenous infusion of carbapenems and pipericillin/tazobactam: A systematic review and meta-analysis. Clin Infect Dis 2013;56(2):272-282. [http://dx.doi.org/10.1093/cid/cis857] [ Links ]
28. Dulhuntty JM, Roberts JA, Davis JS, et al. Continuous infusion of beta-lactam antibiotics in severe sepsis: A multticenter double-blind, randomized controlled trial. Clin Infect Dis 2013;56(2):236-244. [http://dx.doi.org/10.1093/cid/cis856] [ Links ]
 Correspondence:
Correspondence:
M Mer
mervyn.mer@wits.ac.za














