Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SAMJ: South African Medical Journal
versión On-line ISSN 2078-5135
versión impresa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.105 no.1 Pretoria ene. 2015
http://dx.doi.org/10.7196/SAMJ.8185
RESEARCH
Regulation of HIV receptor expression in cervical epithelial cells by Gram-negative bacterial lipopolysaccharide
K J SalesI; T KleinII; A A KatzI
IPhD; MRC/UCT Receptor Biology Research Unit, Institute of Infectious Disease and Molecular Medicine and Division of Medical Biochemistry, Faculty of Health Sciences, University of Cape Town, South Africa
IIBSc (Med) (Hons); MRC/UCT Receptor Biology Research Unit, Institute of Infectious Disease and Molecular Medicine and Division of Medical Biochemistry, Faculty of Health Sciences, University of Cape Town, South Africa
ABSTRACT
BACKGROUND: Sexually transmitted infections (STIs) caused by the Gram-negative bacteria Chlamydia trachomatis and Neisseria gonorrhoeae are associated with an increased risk of HIV acquisition in South African women. HIV infection involves binding of the virus to CD4+ receptors on host cells and subsequent binding to a chemokine co-receptor that mediates fusion with the host target cell membrane.
OBJECTIVE: To investigate the potential impact of STIs on HIV receptor expression in cervical epithelial cells, and the molecular pathways mediating this effect.
METHODS: Expression of Toll-like receptor 4 (TLR4), CD4+ and CCR5 was investigated in HPV type 18-positive (HeLa) and HPV-negative (C33A) cervical epithelial cells, uterine adenocarcinoma cells (Ishikawa), cervical squamous cell carcinoma tissue and normal cervical tissue by real-time polymerase chain reaction (RT-PCR) analysis. HIV receptor expression in HeLa cells was investigated in the presence/absence of 10 μg/mL bacterial lipopolysaccharide (LPS) and chemical inhibitors of epidermal growth factor receptor (EGFR), extracellular signal-regulated kinase (ERK1/2) or cyclo-oxygenase-2 (COX-2) by RT-PCR analysis.
RESULTS: TLR4, CD4+ and CCR5 expression was elevated in HeLa, C33A and Ishikawa cell lines and carcinoma tissue, compared with normal cervical tissue. Treatment of HeLa cells with LPS increased expression of the primary HIV chemokine co-receptor CCR5 (p<0.01) and several alternative HIV receptors including CCR2b (p<0.01), CXCR6 (p<0.05) and GPR1 (p<0.05), but not CD4+. We found that LPS-mediated CCR5 expression occurred via induction of the EGFR, ERK1/2 and COX-2 signalling pathways.
CONCLUSION: Our findings suggest that STIs have the potential to enhance susceptibility to HIV infection in women by regulating expression of HIV receptors in cervical epithelial cells.
Sexually transmitted infections (STIs) with the Gramnegative bacteria Chlamydia trachomatis and Neisseria gonorrhoeae are major public health problems in South Africa (SA) and are significantly associated with HIV infection.[1] In women, STI ultimately occurs at the mucosal surface of the genital tract, where inflammation from both non-ulcerative and ulcerative infections increases localised immune cell mobilisation, in turn enhancing susceptibility to HIV infection.[1] Although HIV preferentially targets CD4+-positive immune cells for infection, recent laboratory studies have shown that cervical epithelial cells can become productively infected and behave as viral reservoirs, sequestering and transferring virus to activated peripheral blood mononuclear cells in the submucosa.[2-5]
HIV infects cells via receptors on the host cell surface. The virus first attaches to the surface of the host cells. The initial step in membrane fusion begins with binding of the viral envelope protein (Env, consisting of a trimer of gp120-gp41 heterodimers) to the CD4+ cell surface protein and a chemokine co-receptor present on the host cell.[6] While most HIV-1 variants use CCR5 and CXCR4 as the main co-receptor in vivo, up to 12 other chemokine co-receptors (including CCR2b, CXCR6 and GPR1) for HIV infection have been identified in vitro.[7,8]
Although the molecular mechanisms regulating HIV chemokine receptors in the cervix are unclear, inflammatory prostaglandins derived by metabolism of arachidonic acid by cyclo-oxygenase enzymes (COX-1 and COX-2)[9] have been shown to regulate HIV chemokine expression in uterine epithelial cells in the female genital tract.'101 These observations suggest that inflammation can drive expression of HIV co-receptors in cervical epithelial cells.
We investigated: (i) the potential impact of Gram-negative bacterial STIs on the regulation of receptors involved in HIV infection in the cervix using the endotoxic component of the Gram-negative bacteria, lipopolysaccharide (LPS), as a mimetic of infection; and (ii) the potential molecular pathways underlying the action of LPS.
Methods
Reagents
Phosphate-buffered saline (PBS) and Tri-reagent* were purchased from Sigma Chemical Company (SA). AG1478, SC560, NS398 and PD98059 were purchased from Calbiochem (Merck, Germany). CCR5 (CKR5; sc-6128) antibody was purchased from Santa Cruz Biotechnology (Whitehead Scientific, SA).
Ethics approval
Ethics approval for the study was obtained from the University of Cape Town Research Ethics Committee (REC/REF: 067/2011). Written informed consent was obtained from all subjects before sample collection.
Tissue collection
Cervical cancer tissue specimens were obtained at the time of surgery or biopsy from patients who were attending the gynaecological oncology clinic at Groote Schuur Hospital (GSH), Cape Town, SA, and had previously been diagnosed with stage 1A moderately differentiated squamous cell carcinoma of the cervix (N=10). The median age of the patients was 41 years. Histologically normal cervical tissue (N=10) was collected from women undergoing Wertheim's hysterectomy for benign gynaecological indications at GSH. The median age of these patients was 50.5 years. Sections of tissue were excised from the ectocervix-transformational zone by a specialist pathologist. Tissue sections were placed into a 15 mL collection tube containing serum-free Dulbecco's Modified Eagle Medium supplemented with 1% penicillin-streptomycin. The tubes were placed on ice and transported to the laboratory. Each sample was divided equally into aliquots before snap-freezing using liquid nitrogen.
Cell culture and treatments
HeLa cells authenticated and verified as cervical adenocarcinoma cells containing HPV type 18 were purchased from Bio-Whittaker (UK). C33A cells were a gift from Prof. Virna Leaner (Division of Medical Biochemistry, University of Cape Town). Ishikawa cells were obtained from the European Collection of Cell Culture (UK). All cell lines were cultured as described previously.[10] For HeLa cell experiments, cells were seeded at a density of 2 x 105 cells in 3 cm dishes and allowed to attach and grow overnight. The following day, cells were serum starved for 24 hours in serum-free medium. Cells were then treated with vehicle (PBS) or 10 μg/mL LPS for 4, 8, 16 or 24 hours. For inhibitor experiments, cells were serum starved and treated with inhibitor alone or 10 μg/mL LPS and inhibitor of epidermal growth factor receptor (EGFR) tyrosine kinase (AG1478; 200 nM), extracellular signal-regulated kinase (ERK1/2) (PD98059; 50 uM), COX-1 (SC-560; 10 uM) or COX-2 (NS398; 10 μM). The concentrations of chemical inhibitors were determined empirically by titration using the IC50 values from the manufacturer as a guide. At the concentrations and time used, the inhibitors showed no adverse effect on cell viability. Fold increase was calculated by dividing the values obtained from the LPS only/ LPS plus inhibitor treatments by the vehicle only/vehicle plus inhibitor treatments.
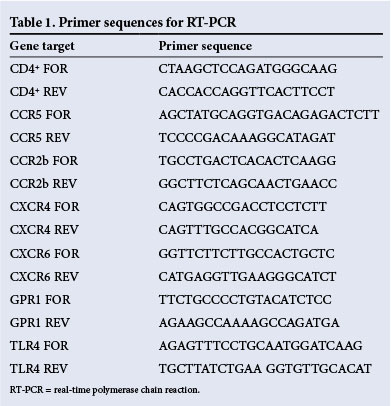
Real-time polymerase chain reaction (RT-PCR) analysis
RNA was extracted using Tri-reagent (Sigma) following the manufacturer's guidelines and reverse transcribed as described previously.[10] All gene expression experiments were carried out on an Illumina Eco™ quantitative RT-PCR machine and detected using SYBR green (Bioline, Celtic Molecular, SA) incorporation during the PCR reaction. Sequences of PCR primers used are outlined in Table 1. A melt curve was performed for each PCR reaction, and all PCR products gave a single peak confirming the purity of the PCR product. Results were calculated using the comparative cycle threshold (Ct) method, and expression of each cDNA sample was normalised for RNA loading using the average Ct value obtained from two independent reference genes (18s ribosomal RNA and glyceraldehyde 3-phosphate dehydrogenase) as internal controls. All data were expressed relative to an endogenous control of HeLa cell cDNA included in each experiment or converted to fold increase, which was determined by dividing the relative expression of the treatment group by the relative expression of each control group. The experiment was conducted in duplicate and data were presented as means (standard error of the mean (SEM)).
Western blot analysis
Cell lysis, protein quantification and immunoblot experiments were conducted as described previously,[11] using a specific CCR5 antibody. CCR5 protein was revealed by chemiluminescence and quantified using a UVP BioSpectrum 500 Imaging System (Scientific Group, SA). The experiment was conducted three times and data were presented as means (SEM).
Statistical analysis
Statistical analysis was performed using one-way analysis of variance and the Newman-Keuls multiple comparison or Dunnett post-hoc test to compare differences in gene expression between the experimental groups. A paired t-test was conducted between vehicle and LPS-treated cells on the untransformed means, before conversion to fold increase. An unpaired t-test was used to compare CCR5 expression in samples treated with LPS in the presence of signalling inhibitors with that in samples treated with LPS only. Analysis and histograms were generated using Graphpad Prism software version 5.00 (Graph Pad, USA). Data were considered significant at p<0.05.
Results
Expression of TLR4, CD4+ and CCR5 in uterine-cervical cells and tissues
We hypothesised that the endotoxic LPS component of the Gramnegative bacteria C. trachomatis and N. gonorrhoeae, often transmitted during sexual intercourse and deposited in the vagina and cervix, could impact on HIV receptor expression in the cervical mucosa. LPS mediates its effect via a signalling receptor, Toll-like receptor 4 (TLR4), to alter signal transduction pathways and increase inflammatory gene expression. We initially investigated the expression of TLR4 in HPV type 18-transformed cervical cancer cells (HeLa), HPV-negative cervical cancer cells (C33A), uterine endometrial adenocarcinoma cells (Ishikawa), cervical squamous cell carcinoma tissue and normal cervical tissue by quantitative RT-PCR analysis.
We found differential expression of TLR4 (Fig. 1, A), CCR5 (Fig. 1, B) and CD4+ (Fig. 1, C) in all cell lines and tissues investigated. TLR4 expression was higher in HeLa cells, Ishikawa cells and cancer tissues than in C33A cells or normal cervical tissue (Fig. 1, A; *p<0.05, **p<0.01). CCR5 (Fig. 1, B; p<0.05) and CD4+ (Fig. 1, C; p<0.05) expression was elevated in cervical cancer tissue compared with normal cervical tissue, but was not significantly different from expression in HeLa cells, C33A cells or Ishikawa cells.
LPS regulates expression of HIV receptors in HeLa cells
Since we had determined that TLR4 was present in cervical tissues and could therefore mediate inflammatory signalling in the cervical mucosa, we next investigated the impact of LPS stimulation on HIV receptor expression in cervical epithelial cells, using HeLa cells as a model system. HeLa cells were treated with vehicle (dark bars in Fig. 2) or LPS (light bars) for 4, 8, 16 and 24 hours, and the RNA was extracted and subjected to reverse transcription and quantitative RT-PCR analysis. We found that CCR5 expression increased in a time-dependent manner, reaching a maximum at 24 hours (Fig. 2, A; p<0.01). We found no significant alteration in expression levels of the main HIV receptor CD4+ in response to LPS treatment at any time point investigated (Fig. 2, B).
HIV-1 and HIV-2 strains have been reported to be capable of utilising several alternative chemokine receptors, including CCR2b, CXCR4, CXCR6 and GPR1, to mediate infection of cells.[7,8] We found that LPS stimulation of HeLa cells increased CCR2b (Fig. 2, C; p<0.01), CXCR6 (Fig. 2, E; p<0.05) and GPR1 (Fig. 2, F; p<0.05) at 4, 8 and 24 hours, respectively, compared with vehicle-treated cells. In contrast, we found no difference in CXCR4 receptor expression in HeLa cells treated with LPS at any time point investigated (Fig. 2, D). These data indicate that LPS could potentially regulate expression of a host of chemokine co-receptors in the cervix, which could mediate infection of cells by HIV strains capable of utilising alternative CD4+-co-receptor combinations, different from CD4+-CCR5 or CD4+-CXCR4.
LPS induces CCR5 receptor expression via the EGFR, COX-2 and ERK1/2 pathways
Since CCR5 is the main receptor utilised by HIV strains for infection, we focused our analysis on this molecule. We have previously highlighted a role for the inflammatory COX-prostaglandin pathway in mediating chemokine receptor expression in the female reproductive tract via the EGFR and ERK1/2 pathways.[10] In the present study, we investigated the role of the EGFR, ERK1/2 and COX pathways in regulating CCR5 expression, using a panel of specific chemical inhibitors of cellular signalling. HeLa cells were treated with vehicle or LPS in the presence/absence of inhibitors of EGFR kinase (AG1478), ERK1/2 (PD98059), COX-1 (SC560) or COX-2 (NS398) for 24 hours, and the mRNA and protein were subjected to quantitative RT-PCR (Fig. 3, A) and Western blot (Fig. 3, B) analysis,
Discussion
Inflammation of the cervical mucosa is considered a significant risk factor for HIV infection.[1] However, the roles of inflammatory mediators and STIs in regulating pathways involved in HIV infection in the cervix have yet to be fully elucidated. The endotoxic component of Gram-negative bacteria, LPS, is found on the outer bacterial membrane and is responsible for eliciting strong immune responses, associated with infection, by binding to and activating TLR4.[12] We hypothesised that this endotoxic component of Gram-negative bacteria, which is abundantly part of the make-up of C. trachomatis and N. gonorrhoeae, could regulate chemokine receptors and pathways with known roles in HIV infection in cervical epithelial cells to enhance susceptibility of the genital tract to infection.
To investigate whether the endotoxic component of Gram-negative bacteria, LPS, could have an impact on cervical epithelial cells, we screened several uterine-cervical epithelial cell lines, cervical squamous cell carcinomas and normal cervical cells to determine whether TLR4 was present. We found that all cell lines and tissues expressed TLR4, the signalling receptor for LPS, as well as the main HIV receptors CD4+ and CCR5. These findings indicated that all cell lines and tissues expressed the necessary cellular machinery to mediate infection of the cervix by Gram-negative bacteria and HIV.
The epithelial compartment of several tissues in the human body, including the gastrointestinal tract, prostate and cervix, has been implicated in the uptake and transport of HIV to submucosal leucocytes.[5,13] Of significance to our study is the observation that cervical epithelial cells can behave as viral reservoirs, to sequester and transfer virus to activated peripheral blood mononuclear cells in the submucosa.[2,5,14] Moreover, several studies have shown that levels of CCR5 in cells positively correlate with HIV infectivity and levels of cellular activation in vivo.[4,15,16]
It is therefore plausible that any mechanism that enhances CCR5 expression, or indeed expression of other alternative HIV co-receptors such as CCR2b, CXCR6 and GPR1 investigated in this study, could enhance HIV susceptibility. In sexually active women, this could be enhanced by bacterial STIs mediated by C. trachomatis and N. gonorrhoeae. These agents of infection could mediate HIV infection both directly by regulating cell surface expression of chemokine G protein-coupled receptors to mediate virus fusion and infection and indirectly by facilitating the recruitment of CD4+-positive immune cells into the local cervical environment, which could then be targeted by HIV for infection.
Exploring the intracellular pathways mediating the induction of CCR5 by LPS, we found that LPS regulates CCR5 mRNA and protein expression in HeLa cells via the EGFR, ERK1/2 and inflammatory COX-2 pathways. Many pathological disorders or diseases, including cervical cancer, have been characterised by the exacerbated activation and maintenance of these inflammatory pathways.'171 Over the past two decades, significant attention has been paid to inhibition of the inflammatory COX enzyme pathway as a potential therapeutic intervention strategy for a host of inflammatory diseases. Our observations of the role of COX-2 in regulating CCR5 expression suggest that administration of non-steroidal anti-inflammatory drugs such as aspirin to suppress COX-2 expression in sexually active women with lower urinary tract bacterial infections might also suppress inflammatory pathways that regulate HIV receptor expression and susceptibility to HIV infection.
Our study shows that the endotoxic LPS component of bacterial STIs, which are very common in sexually active women, often in the absence of any symptoms of infection, can regulate expression of HIV receptors in the cervical epithelium. Since levels of HIV receptor positively correlate with HIV infectivity, and since the cervical mucosa is known to become productively infected by virus, respectively. We found that AG1478, PD98059 and NS398, but not SC560, significantly inhibited the LPS-mediated induction of CCR5 mRNA (Fig. 3, A) and protein (Fig. 3, B) in HeLa cells (*p<0.05, **p<0.01).
our data highlight the potential of STIs for enhancing the risk of infection by HIV during intercourse, by increasing the abundance of cell surface machinery used by the HI virus for establishment of infection.
Sources of funding. This study was supported by grant funding to the MRC/UCT Receptor Biology Research Unit by the Medical Research Council of South Africa and by the following grants to KJS: Poliomyelitis Research Foundation of South Africa, Cancer Association of South Africa, National Research Foundation of South Africa and University of Cape Town Research Committee. The funders played no role in the conception or design of the study, the interpretation of the results or the decision to publish.
References
1. Pham-Kanter GB, Steinberg MH, Ballard RC. Sexually transmitted diseases in South Africa. Genitourin Med 1996;72(3):160-171. [ Links ]
2. Dezzutti CS, Guenthner PC, Cummins JE Jr, et al Cervical and prostate primary epithelial cells are not productively infected but sequester human immunodeficiency virus type 1. J Infect Dis 2001;183(8):1204-1213. [http://dx.doi.org/10.1086/319676] [ Links ]
3. Maher D, Wu X, Schacker T, et al. HIV binding, penetration, and primary infection in human cervicovaginal tissue. Proc Natl Acad Sci U S A 2005;102(32):11504-11509. [http://dx.doi.org/10.1073/pnas.0500848102] [ Links ]
4. Wu L, Paxton WA, Kassam N, et al. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med 1997;185(9):1681-1691. [http://dx.doi.org/10.1084/jem.185.9.1681] [ Links ]
5. Wu Z, Chen Z, Phillips DM. Human genital epithelial cells capture cell-free human immunodeficiency virus type 1 and transmit the virus to CD4+ cells: Implications for mechanisms of sexual transmission. J Infect Dis 2003;188(10):1473-1482. [http://dx.doi.org/10.1086/379248] [ Links ]
6. Wilen CB, Tilton JC, Doms RW. Molecular mechanisms of HIV entry. Adv Exp Med Biol 2012;726:223-242. [http://dx.doi.org/10.1007/978-1-4614-0980-9_10] [ Links ]
7. Doranz BJ, Lu ZH, Rucker J, et al. Two distinct CCR5 domains can mediate coreceptor usage by human immunodeficiency virus type 1. J Virol 1997;71(9):6305-6314. [ Links ]
8. Shimizu N, Tanaka A, Oue A, et al. Broad usage spectrum of G protein-coupled receptors as coreceptors by primary isolates of HIV. AIDS 2009;23(7):761-769. [http://dx.doi.org/10.1097/QAD.0b013e328326cc0d] [ Links ]
9. Rizzo MT. Cyclooxygenase-2 in oncogenesis. Clin Chim Acta 2011;412(9-10):671-687. [http://dx.doi.org/10.1016/j.cca.2010.12.026] [ Links ]
10. Sales KJ, Grant V, Catalano RD, et al. Chorionic gonadotrophin regulates CXCR4 expression in human endometrium via E-series prostanoid receptor 2 signalling to PI3K-ERK1/2: Implications for fetal-maternal crosstalk for embryo implantation. Mol Hum Reprod 2011;17(1):22-32. [http://dx.doi.org/10.1093/molehr/gaq069] [ Links ]
11. Sales KJ, Katz AA, Howard B, et al. Cyclooxygenase-1 is up-regulated in cervical carcinomas: autocrine/ paracrine regulation of cyclooxygenase-2, prostaglandin e receptors, and angiogenic factors by cyclooxygenase-1. Cancer Res 2002;62(2):424-432. [ Links ]
12. Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol 2010;64:163-184. [http://dx.doi.org/10.1146/annurev.micro.091208.073413] [ Links ]
13. Meng G, Wei X, Wu X, et al. Primary intestinal epithelial cells selectively transfer R5 HIV-1 to CCR5+ cells. Nat Med 2002;8(2):150-156. [http://dx.doi.org/10.1038/nm0202-150] [ Links ]
14. Gupta P, Collins KB, Ratner D, et al. Memory CD4(+) T cells are the earliest detectable human immunodeficiency virus type 1 (HIV-1)-infected cells in the female genital mucosal tissue during HIV-1 transmission in an organ culture system. J Virol 2002;76(19):9868-9876. [http://dx.doi.org/10.1128/JVI.76.19.9868-9876.2002] [ Links ]
15. Stoddart CA, Keir ME, McCune JM. IFN-alpha-induced upregulation of CCR5 leads to expanded HIV tropism in vivo. PLoS Pathog 2010;6(2):e1000766. [http://dx.doi.org/10.1371/journal.ppat.1000766] [ Links ]
16. Ostrowski MA, Justement SJ, Catanzaro A, et al. Expression of chemokine receptors CXCR4 and CCR5 in HIV-1-infected and uninfected individuals. J Immunol 1998;161(6):3195-3201. [ Links ]
17. Sales KJ, Katz AA. Inflammatory pathways in cervical cancer - the UCT contribution. S Afr Med J 2012;102(6):493-496. [ Links ]
 Correspondence:
Correspondence:
K J Sales
kurtjsales@gmail.com
Accepted 10 November 2014.














