Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.105 n.1 Pretoria Jan. 2015
http://dx.doi.org/10.7196/SAMJ.8419
RESEARCH
The Vaccine and Cervical Cancer Screen (VACCS) project: Acceptance of human papillomavirus vaccination in a school-based programme in two provinces of South Africa
M H BothaI; F H van der MerweII; L C SnymanIII; G DreyerIV
IMB ChB, MMed (O&G), FCOG (SA), PhD; Department of Obstetrics and Gynaecology and Unit for Gynaecological Oncology, Faculty of Medicine and Health Sciences, Stellenbosch University, Tygerberg, Cape Town, South Africa
IIMB ChB, MMed (O&G), FCOG (SA);Department of Obstetrics and Gynaecology and Unit for Gynaecological Oncology, Faculty of Medicine and Health Sciences, Stellenbosch University, Tygerberg, Cape Town, South Africa
IIIMB ChB, MPraxMed, MMed (O&G), FCOG (SA); Department of Obstetrics and Gynaecology and Gynaecological Oncology Unit, Faculty of Health Sciences, University of Pretoria, South Africa
IVMB ChB, MMed (O&G), MCOG (SA), PhDDepartment of Obstetrics and Gynaecology and Gynaecological Oncology Unit, Faculty of Health Sciences, University of Pretoria, South Africa
ABSTRACT
BACKGROUND: The incidence of cervical cancer in South Africa (SA) remains high, and the current screening programme has had limited success. New approaches to prevention and screening tactics are needed.
OBJECTIVES: To investigate acceptance of school-based human papillomavirus (HPV) vaccination, as well as the information provided, methods of obtaining consent and assent, and completion rates achieved.
METHODS: Information on cervical cancer and HPV vaccination was provided to 19 primary schools in Western Cape and Gauteng provinces participating in the study. Girls with parental consent and child assent were vaccinated during school hours at their schools.
RESULTS: A total of 3 465 girls were invited to receive HPV vaccine, of whom 2 046 provided written parental consent as well as child assent. At least one dose of vaccine was delivered to 2 030 girls (99.2% of the consented cohort), while a total of 1 782 girls received all three doses. Sufficient vaccination was achieved in 91.6% of the vaccinated cohort. Of all invited girls, 56.9% in Gauteng and 50.7% in the Western Cape were sufficiently vaccinated.
CONCLUSION: This implementation project demonstrated that HPV vaccination is practical and safe in SA schools. Political and community acceptance was good, and positive attitudes towards vaccination were encountered. During the study, which mimicked a governmental vaccine roll-out programme, high completion rates were achieved in spite of several challenges encountered.
Cervical cancer remains an important cause of morbidity and mortality in South Africa (SA).[1] The age-standardised incidence rate of cervical cancer in southern Africa is approximately 27/100 000,[2] and most cases are diagnosed in late stages. Persistent infection with oncogenic human papillomavirus (HPV) is an essential step in the development of invasive cervical cancer.[3] HPV is highly infectious, but does not cause disease in all cases, and most individuals will clear infections. Since HPV is almost exclusively an epithelial disease, most transient HPV infections do not confer long-term immunity owing to a poor immune response.
A national cervical cancer prevention programme was launched in SA in 2000, offering three Papanicolaou smears in a woman's lifetime, starting after the age of 30 at 10-year intervals, but has had limited success in reducing the incidence of HPV-associated disease. Some provinces in SA have fairly well-developed cytology screening services, but there is poor uptake of prevention services for cancer. Among women with abnormal cytology, there is also significant loss to follow-up after the initial screening test.[4]
Since the introduction of effective HPV vaccines, a primary preventive strategy became available to combat the epidemic. Currently there are two vaccines registered in SA: the bivalent vaccine Cervarix, containing virus-like particles (VLPs) for HPV types 16 and 18, and the quadrivalent vaccine Gardasil, containing VLP antigens for HPV types 16 and 18 as well as non-oncogenic HPV types 6 and 11. VLPs are combined with an adjuvant, which leads to an improved immune response and long-term efficacy. Both vaccines have been extensively tested in large populations, and have also been found to be safe and immunogenic among African populations.[5] The bivalent HPV vaccine has also shown sustained immune responses in HIV-positive women, and was well tolerated.[6] Local reactions such as pain, swelling and redness can occur, as may systemic adverse events including fever, nausea, dizziness, fatigue, headache and myalgia.
Cost-effectiveness studies have shown universal, female-only HPV vaccination before exposure to be an effective and economically viable option in developed countries.[7] Recently there has been increasing emphasis on the inclusion of low- and middle-income countries in the drive to reduce the global cancer burden. Evidence from qualitative studies suggests that South Africans will support introduction of HPV vaccination, but that education remains a key ingredient in any roll-out.[8] Adolescent health was identified as an area for development in the SA National Health Initiative Green Paper, with preventive health an important part of this plan. This focus is linked to a re-engineered primary healthcare plan and a newly developed school health programme (SHP).
In May 2013, Dr Aaron Motsoaledi, national Minister of Health, announced during the health budget speech that: '... we shall commence to administer the HPV vaccines as part of our SHP ...'. This courageous step is widely applauded in view of an uncontrolled cervical cancer epidemic resulting from high prevalence rates of HPV and HIV and the relatively unsuccessful cervical cancer screening programme described above. The success of this programme will depend on offering the vaccine to the target group via a functional SHP, education, and high vaccine uptake and completion rates. Data on factors influencing successful school-based implementation as well as acceptance rates among SA primary schoolgirls and their parents are limited.
This paper describes recruitment, information provided and consent and assent methods used, and reports on the acceptance of HPV vaccination and completion rates among learners invited to receive HPV vaccination in a primary school-based programme.
Methods
Approval
Approval to conduct the project in schools was obtained from the national and provincial departments of health and education. Ethics approval was received from the participating university human research ethics committees (Stellenbosch N11/01/008 and Pretoria 219/2009). There was discussion between the investigators and school principals, important teachers and the school governing bodies to explain the rationale of the work.
Study procedures
Selection of sites and participants
Primary schools in low socioeconomic areas in Western Cape and Gauteng provinces were identified and contacted to invite them to participate in this prospective demonstration study. Children in grades 4 - 7 received an invitation letter to an information evening to take home.
Information, consent and assent procedures
Teachers encouraged learners to bring the reply slip back. An information event was scheduled at each school in the afternoon or early evening. At the information events parents were interviewed after informed consent for the interview had been obtained. Structured interviews were conducted by trained interviewers and lasted about 20 minutes. The interview focused on demographic information and knowledge and perceptions of cervical cancer screening and vaccination. After the interviews, information about cervical cancer and ways of preventing it, including vaccination and screening, was given to parents and learners. The parents were then given the opportunity to provide written consent for vaccination of their children. Children were asked to give written assent as well, in order to meet research ethics requirements. Children aged 12 years and older were legally capable of giving consent without parental consent. The parents were invited to take part in a screening programme.
Parents who did not attend information events were given the opportunity to give consent after written information leaflets were sent home with their children. These consent forms were collected by teachers before the vaccination date.
Vaccine procedures
Vaccines for the study were donated by both vaccine companies. For practical reasons, the majority of girls in a particular school received the same brand of vaccine. All children younger than 10 years received Gardasil, owing to licensing at the time of the study. Vaccination was performed and recorded by registered nurses during school hours.
Schools were visited on at least two occasions for each vaccination time point to account for absenteeism. Learners were observed for 20 minutes after each dose for possible early adverse events.
Statistical analysis
For the purposes of this analysis, we defined the invited cohort (IC) as all female learners, aged 9 years and older, enrolled in grades 4, 5, 6 and 7. The consented cohort (CC) was defined as all participants who had written parental consent as well as assent from the learner herself. Girls with consent who did not receive the vaccine were included in the CC. The vaccinated cohort (VC) included all girls who received at least one dose of vaccine.
Consent rate was calculated as the CC as a proportion of the IC. Vaccine uptake rates were calculated[9] in a number of ways to allow comparison with previously published HPV vaccine reports. The consented uptake rate was calculated as the VC as a proportion of the CC, and the invited uptake rate was calculated as the VC as a proportion of the IC.
Vaccine completion was calculated using the VC as denominator, while sufficient vaccination was calculated using the IC as denominator. When two vaccine doses were administered, data for girls receiving the two doses within a short period of time (<6 months apart) were separated from those who received the vaccines at least 6 months apart. For the purposes of these calculations, girls who received at least two doses 6 months apart were considered sufficiently vaccinated, while those receiving no dose at 6 months were considered insufficiently vaccinated. This is based on recent data suggesting protective antibody levels against vaccine HPV types in similar recipients.[10]
Results
The number of girls of the target age enrolled in the schools approached was 3 465. A total of 2 046 girls had written parental consent as well as written child assent, and 2 030 girls received at least one dose. These cohorts, shown in Table 1, were used to calculate vaccination success rates.
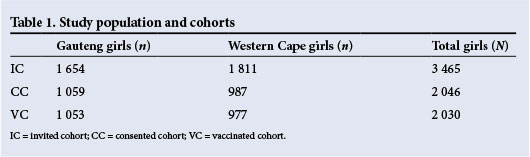
The rate of consent for the total study population was 59.0%. We were able to confirm the consent documents of 87.5% of all parents who attended the information events in Gauteng. Similar data for the Western Cape were not available. Of the consented children, almost all (99.2%) received at least one dose of vaccine. When vaccine uptake rates were calculated for the total target population, we obtained rates of 63.7% for the Gauteng schools and 53.9% in the Western Cape. Recruitment documents and methods were identical for the two provinces, and the reasons for the observed difference in the rate of vaccine uptake were probably independent of the study method. These figures are set out in Table 2.

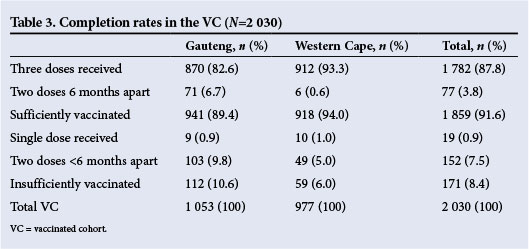
Regarding vaccine completion rates, in the Western Cape 93.3% of all girls who started vaccination received all three doses, and in Gauteng this figure was 82.6%. This difference in completion rates is attributed in part to two schools in Gauteng where the third vaccine dose was scheduled for the following calendar year. In the total group, 91.6% of vaccinated girls were considered sufficiently vaccinated, the figure again being higher in the Western Cape than in Gauteng. Completion rates are set out in Table 3.
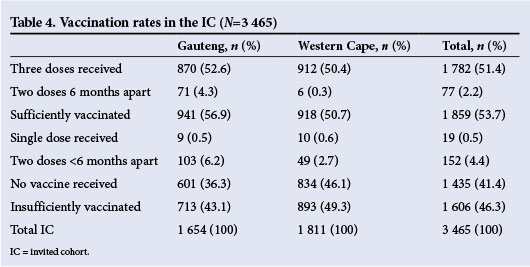
Considering both the influences of vaccine uptake and doses received, 53.7% of the target population in this study was considered sufficiently vaccinated at the end of the study period. Another 4.9% were vaccinated, but received a suboptimal vaccine dosage -usually the first two doses not followed by a third dose 6 months after the first dose. Detailed data are presented in Table 4.
Vaccination proceeded without any serious side-effects, and no serious adverse events were reported. There was protocol violation in two instances where two girls received one dose each without full written consent.
Discussion
In general, the staff at the schools co-operated and supported the study. At the majority of schools, staff members helped with the information evenings. Attendance at these meetings varied from school to school and according to seasons, television schedules and safety concerns. Most of the parents who attended the information events gave consent for vaccination of their children, but a significant percentage did not attend these events and did not reply to the written invitation to take part in the study. The successful Australian school-based programme sends information to parents and asks for a detailed consent form to be returned to the school.[1] Information leaflets were also used to communicate with parents in a demonstration project in another province of SA, but the authors do not mention the number of children approached or the proportion for whom parental consent was achieved.[12]
Information leaflets may work in certain schools, but our experience was that about 30 - 40% of parents did not respond to letters sent home with the learners. In the current study, relatively limited written information was provided to parents, but written information was combined with an invitation to an information event at which detailed verbal information was given and opportunity for questions and discussion provided. Using this recruitment method, a consent rate of almost 90% was reached among parents attending the information event, v. a rate of almost 60% for the total IC.
The high acceptance rate following better information underlines the importance of clear, direct communication with parents. In Rwanda a high rate of acceptance was achieved after educational interventions to parents.[13] There seems to be a better response to vaccination uptake in certain populations when interactive communication (rather than written only) strategies are used for transfer of information.[14] Verbal, interactive information sessions may be key to success in areas where literacy levels are low.
Uptake rates in many HPV vaccination projects around the world have been reported as high.[1,12] In our CC, 99.4% of girls were vaccinated and 91.6% received at least two doses of vaccine 6 months apart. Another 7.5% received their second dose after the first without receiving the last, while 87.8% received all three doses. Lower uptake was achieved when all invited girls are considered, but this is difficult to compare with other studies owing to a lack of local data.[12]
Linking primary and secondary prevention in a mother-daughter programme was tested in this study and other sites.[12,15] Mothers and/or caregivers of learners were given the opportunity to undergo screening. The results of this part of the study will be reported in the next issue of the SAMJ.[9]
Conclusion
This implementation project demonstrated that HPV vaccination is practical and safe in SA schools. Political and community acceptance is good, and we encountered positive attitudes towards vaccination. This study mimicked a governmental vaccine roll-out programme, and despite several challenges, high completion rates were achieved.
Parents who received complete information on the HPV vaccine demonstrated a very high acceptance rate, appropriate information contributing significantly to vaccine uptake. This effective communication is shown to achieve better coverage for vaccination; importantly, the awareness created may also lead to improved screening uptake. Vaccine completion was much improved by ensuring administration of all doses within a single calendar or academic year.
Acknowledgements. The assistance of the following groups and persons that enabled this project to be completed successfully is gratefully acknowledged.
Financial support was received from the Cancer Research Initiative of South Africa, a national collaborative research programme supported by the South African Medical Research Council, the Cancer Association of South Africa and First for Women Insurance for screening, treatment of screen-positive women and investigator support. The vaccine manufacturing companies GlaxoSmithKline/Aspen SA and Merck supported this investigator-initiated study by generously donating all vaccines used in the project.
Prof. Gerhard Lindeque provided valuable advice, Ms Bertha Grond managed the finances, Ms Riekie Burden, Sr Nicolene Laubscher, Sr Nadine Chamberlain and their teams of registered nurses handled study and vaccine processes, Dr Karin Richter managed the laboratory screening data, Ms Cathy Visser collated and analysed the vaccination and screening data, consultants and registrars in the Department of Obstetrics and Gynaecology, University of Pretoria, presented lectures at the information events, and undergraduate medical students at the University of Pretoria and Stellenbosch University administered the questionnaires.
References
1. ICO (Institut Català d'Oncologia) Information Centre on HPV and Cancer. South Africa: Human Papillomavirus and Related Cancers, Fact Sheet 2013. http://www.hpvcentre.net/statistics/reports/ZAF_FS.pdf (accessed 24 March 2014). [ Links ]
2. Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyons, France: International Agency for Research on Cancer, 2013. http://globocan.iarc.fr (accessed 28 March 2014). [ Links ]
3. Stanley M. Prevention strategies against the human papillomavirus: The effectiveness of vaccination. Gynecol Oncol 2007;107(2):S19-S23. [http://dx.doi.org/10.1016/j.ygyno.2007.07.068] [ Links ]
4. Cronje HS, Beyer E. Screening for cervical cancer in the developing world. Best Pract Res Clin Obstet Gynaecol 2005;19(4):517-529. [http://dx.doi.org/10.1016/j.ijgo.2003.09.009] [ Links ]
5. Sow PS, Watson-Jones D, Kivia N, et al. Safety and immunogenicity of human papillomavirus-16/18 AS04-adjuvanted vaccine: A randomized trial in 10 - 25-year-old HIV-seronegative African girls and young women. J Infect Dis 2013;207(11):1753-1763. [http://dx.doi.org/10.1093/infdis/jis619] [ Links ]
6. Denny L, Hendricks B, Gordon C, et al. Safety and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine in HIV-positive women in South Africa: A partially-blind randomised placebo-controlled study. Vaccine 2013;31(48):5745-5753. [http://dx.doi.org/10.1016Zj.vaccine.2013.09.032] [ Links ]
7. Anonychuk AM, Bauch CT, Merid ME, van Kriekinge G, Demarteau N. A cost-utility analysis of cervical cancer vaccination in preadolescent Canadian females. BMC Public Health 2009;9:401. [http://dx.doi.org/10.1186/1471-2458-9-401] [ Links ]
8. Harries J, Moodley J, Barone MA, Mall S, Sinanovic E. Preparing for HPV vaccination in South Africa: Key challenges and opinions. Vaccine 2009;27(1):38-44. [http://dx.doi.org/10.1016/j.vaccine.2008.10.033] [ Links ]
9. Snyman LC, Dreyer G, Botha MH, van der Merwe FH, Becker PJ. The Vaccine and Cervical Cancer Screen (VACCS) project: Linking cervical cancer screening to HPV vaccination in the South West Tshwane District of Gauteng. S Afr Med J 2015 (in press). [http://dx.doi.org/10.7196/SAMJ.8418] [ Links ]
10. Dobson SRM, McNeil S, Dionne M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: A randomized clinical trial. JAMA 2013;309(17):1793-17802. [http://dx.doi.org/10.1001/jama.2013.1625] [ Links ]
11. Australian Government Department of Health. HPV school vaccination program. 2014. http://hpv.health.gov.au/the-program/#.UzlD_9LHlic (accessed 31 March 2014). [ Links ]
12. Moodley I, Tathiah N, Mubaiwa V, Denny L. High uptake of Gardasil vaccine among 9 - 12-year-old schoolgirls participating in an HPV vaccination demonstration project in KwaZulu-Natal, South Africa. S Afr Med J 2013;103(5):318-321. [http://dx.doi.org/10.7196/SAMJ.6414] [ Links ]
13. Binagwaho A, Wagner CM, Gatera M, Karema C, Nutt CT, Ngaboa F. Achieving high coverage in Rwanda's national human papillomavirus vaccination programme. Bull World Health Organ 2012;90(8):623-628. [http://dx.doi.org/10.2471/BLT.11.097253] [ Links ]
14. Galagan SR, Paul P, Menezes L, LaMontagne DS. Influences on parental acceptance of HPV vaccination in demonstration projects in Uganda and Vietnam. Vaccine 2013;31(30):3072-3078. [http://dx.doi.org/10.1016/j.vaccine.2013.04.056] [ Links ]
15. Abuelo CE, Levinson KL, Salmeron J, Sologuren CV, Fernandez MJV, Belinson JL. The Peru Cervical Cancer Screening Study (PERCAPS): The design and implementation of a mother/daughter screen, treat, and vaccinate program in the Peruvian jungle. J Community Health 2013;39(3):409-415. [http://dx.doi.org/10.1007/s10900-013-9786-6] [ Links ]
 Correspondence:
Correspondence:
M H Botha
mhbotha@sun.ac.za
Accepted 10 November 2014.














