Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.104 n.12 Pretoria Dec. 2014
http://dx.doi.org/10.7196/SAMJ.9097
CONTINUING MEDICAL EDUCATION
ARTICLE
Diagnosis and management of drug-resistant tuberculosis in South African adults
J HughesI; M OsmanII
IMB BCh (Cardiff); Médecins sans Frontieres (MSF, Doctors without Borders), Khayelitsha, Cape Town, South Africa
IIMB ChB, PG Dip Health Management City Health, Cape Town, South Africa
ABSTRACT
Detection of drug-resistant tuberculosis (DR-TB) increases each year in South Africa (SA). Most cases result from airborne transmission of already resistant TB strains. Epidemic control relies on rapid diagnosis and initiation of effective treatment to reduce the period of infectiousness and ongoing transmission. The rapid diagnostic test, Xpert MTB/RIF, has replaced smear microscopy for routine screening of all cases of presumptive TB in SA. Xpert also detects rifampicin (RIF) resistance, an indicator of more extensive drug resistance, allowing rapid initiation of effective second-line treatment. Definitive diagnosis of DR-TB relies on laboratory confirmation of MTB, along with drug-susceptibility testing (DST) using culture-based (phenotypic) and/or molecular (genotypic) techniques. A standardised treatment regimen, consisting of five (or six) drugs (pyrazinamide, (ethambutol), kanamycin, moxifloxacin, ethionamide, terizidone), is offered to individuals following initial diagnosis of RIF resistance. Treatment regimens are individualised if and when molecular mutation details and second-line DST results indicate more extensive second-line drug resistance. DR-TB treatment outcomes are poor owing to death, and interruption and failure of current treatment. Reliable access to newer, more effective drugs within shorter, more tolerable regimens is desperately needed to improve the chance of a cure for DR-TB patients.
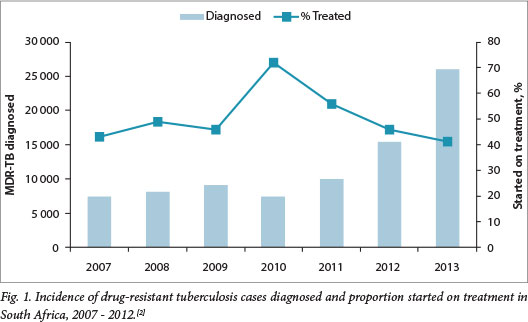
Drug-resistant tuberculosis (DR-TB) is caused by Mycobacterium tuberculosis (MTB) with demonstrated resistance to first-line and/or second-line TB drugs on laboratory drug-susceptibility testing (DST). The revised classification and definitions of DR-TB, according to the World Health Organization (WHO), are given in Table Rifampicin (RIF), one of the most potent first-line TB drugs, results in sterilisation and has allowed the duration of TB treatment to be shortened to 6 months with low risk of subsequent relapse. Therefore, any RIF-resistant TB requires treatment with second-line TB drugs for 18 months - 2 years. Detection of resistance to RIF is an important indicator for more extensive resistance to other first-line drugs in countries where prevalence is high.[2] Approximately 1.8% of new TB cases and 6.7% of previously treated TB cases in SA are multidrug resistant (MDR).[2] MTB may acquire resistance to certain drugs through selective drug pressure from suboptimal treatment,[3] such as in situations where adherence is poor. However, the majority of new DR-TB cases diagnosed in SA are due to transmission of already resistant strains.[4] Unpublished data in annual reports from the Western Cape Department of Health indicate that half of all new DR-TB cases diagnosed each year have never received TB treatment. Significant scale-up of access to services, with widespread implementation of rapid diagnostic techniques and prompt initiation of effective treatment in all diagnosed patients, is required to reduce ongoing DR-TB transmission. Fig. 1 demonstrates a rising DR-TB case detection, with a widening gap between those diagnosed and those treated in SA in recent years.[2] The National Department of Health has addressed this by endorsing a policy of decentralisation of DR-TB services to allow MDR treatment initiation at designated decentralised units across the country.[5] With appropriate training and support this is also feasible at a primary care level, as demonstrated in Khayelitsha, Western Cape Province.[6]
Diagnosis of DR-TB
Owing to the high prevalence of DR-TB in SA, all patients with presumptive TB should ideally be screened for at least RIF resistance at initial clinical presentation. The signs, symptoms and radiographic features are indistinguishable from those of drug-sensitive TB and therefore phenotypic and/ or genotypic laboratory tests are required for definitive diagnosis. Table 2 gives an overview of the available diagnostic tests.
Molecular (or genotypic) techniques based on nucleic acid amplification can simplify logistics and reduce laboratory workload and are now used routinely in SA for identification of MTB, as well as rapid detection of genetic mutations associated with resistance to selected drugs. Direct smear microscopy has largely been replaced by the Xpert MTB/RIF (Cepheid, Sunnyvale, USA) test for routine detection of MTB in SA, but smear is still useful in confirmed TB cases to monitor bacterial load and response to treatment in individuals who produce smear-positive sputum initially. Xpert MTB/ RIF, or GeneXpert (GXP), was endorsed by the WHO in 2010; this technique identifies MTB and RIF resistance associated with specific mutations in the rpoB gene through an automated real-time polymerase chain reaction (PCR) technique.[7] This cartridge-based closed system reduces both the infectious risk to laboratory workers and the risk of PCR contamination. The test takes <2 hours and, although not quite point of care, may be used in any laboratory with trained personnel. Following the nationwide roll-out of Xpert instruments in 2011, the national TB guidelines recommend an algorithmic approach for using GXP in new cases of presumed TB.[8] As the GXP identifies genetic material, which includes non-viable mycobacteria, it is less reliable for diagnosis of active TB in patients who completed TB treatment recently or for monitoring those already on TB treatment.
Following the detection of RIF-resistant TB using GXP, further testing is necessary to confirm RIF resistance and allow for more extensive DST. Definitive diagnosis of MTB is still through growth (and subsequent molecular identification) of viable bacilli on solid or liquid culture media, but because of the variable and inherent slow growth of the mycobacteria it takes 6 weeks for the culture to be reported negative.[9] Genotypic tests, such as the GenoType MTBDRplus (Hain Lifescience, Nehren, Germany) line-probe assay (LPA), can be done directly on smear-positive samples to rapidly confirm MTB and detect resistance to RIF and/or isoniazid (INH), but a culture is still necessary in the large number of smear-negative TB cases (especially common with HIV co-infection) for the LPA to be carried out on the cultured isolate. While the specificity of the LPA is the same for detection of RIF and INH resistance (99%), sensitivity is higher for detection of RIF (97%) than INH resistance (90%).[10] Therefore, phenotypic DST should be carried out routinely for confirmation of INH susceptibility in cases reported as RIF monoresistant on LPA. The majority of RIF resistance cases are due to a mutation in the rpoB gene, but the LPA reports resistance to INH based on mutations in two different genes, inhA and KatG. For INH there may be discrepancies between mutations detected on molecular testing and phenotypic DST. n] While mutations in the KatG gene are likely to confer high-level INH resistance, mutations in the inhA gene may confer lower-level resistance and be overcome by higher doses of INH (16 - 18 mg/kg).[12] Additionally, mutations in the inhA gene may also confer cross-resistance to ethionamide (Eto),[11] one of the main second-line drugs used in the standard treatment of MDR-TB.
Second-line DST for amikacin and ofloxacin is carried out routinely for all RIF-resistant samples using phenotypic culture-based methods to determine the growth of MTB bacilli in the presence of individual drugs. These results only become available weeks or months following initiation of MDR treatment and should be actively followed up as detection of further resistance represents extensively drug-resistant (XDR) or pre-XDR TB and necessitates modification of treatment. The GenoType MTBDRs/ (Hain Lifescience, Nehren, Germany) rapidly detects mutations conferring resistance to second-line drugs (specifically fluoroquinolones, ethambutol (EMB) and the injectable agents); however, concerns over the sensitivity of the test, particularly for resistance to the injectables, have delayed implementation in the routine diagnostic algorithm and the test is undergoing further evaluation.
Discordance between GXP, LPA and/or phenotypic test results is not uncommon, and may cause confusion and delay treatment initiation. This highlights the importance of sending a second sample for confirmation of positive GXP results, and ideally a third sample to be taken at treatment initiation for culture and DST to provide further confirmation in cases of initial discordance. In patients with discordant results, correlation with clinical findings is crucial and specialist consultation may be necessary to ensure that they receive the most appropriate treatment without adversely affecting their chance of cure, but simultaneously limiting exposure to unnecessary and toxic drugs.
Treatment of DR-TB
All patients with an initial diagnosis of DR-TB in SA are offered a standardised treatment regimen, which may be individually tailored at a later stage if and when further DST results become available. The standard regimen is usually designed at a national or regional level based on drug resistance data from prevalence surveys in representative patient populations; the public health benefit of this approach is wider and there is more rapid access to effective treatment, which is not initially reliant on specialist knowledge or skills. Table 3 describes the step-wise approach to designing regimens from the five WHO categories of TB drugs.[3] The WHO recommends at least four possible effective drugs initially, with use of the injectable agent for a minimum of 8 months, and a total treatment duration of 20 months.[3] The standardised regimen in SA comprises at least five drugs (EMB is sometimes included depending on treatment history and local prevalence of EMB resistance): pyrazinamide/ethambutol/kanamycin/ moxifloxacin/ethionamide/terizidone.
Weight-based dosing, common side-effects and recommended monitoring for each drug are given in Table 4. The current SA DR-TB guidelines advise at least 6 months for the injectable phase, until 4 months post-sputum culture conversion (date of first of two negative sputum cultures taken at least 30 days apart), with total treatment duration calculated up to at least 18 months from the date of culture conversion.[13]
Unfortunately, clinical outcomes remain poor in SA, with <45% of MDR-TB cases reported as treatment success (cure or completion).[2] The current standard MDR regimen is toxic and lengthy, with potentially fewer than four possible effective drugs, and therefore should be optimised where possible. DST for pyrazinamide (PZA) and EMB is not carried out routinely owing to poor reliability of the available testing methods, but genotyping studies have estimated that roughly 50% of MDR strains in SA are susceptible to PZA and/ or EMB and therefore routine addition to the standard regimen may benefit half of all MDR cases.[14] Although generally well tolerated, these two drugs may be more easily discontinued if there are concerns over pill burden or poor tolerance as they are not relied on as being effective. Terizidone is used despite lack of good evidence for efficacy and no routine DST for this drug. The presence of mutations in the inhA gene potentially indicates cross-resistance to Eto. If INH mutation data are routinely reported, clinicians could modify the regimen to remove Eto and add higher doses of INH (16 - 18 mg/kg) in cases with inhA mutations only. An algorithmic approach has been implemented in the Western Cape, whereby patients with GXP RIF-resistant TB are routinely offered a 7-drug regimen, which is later modified to withdraw one or more drugs according to information subsequently obtained from LPA results (Fig. 2). In a minority of situations where both inhA and KatG mutations are present (precluding use of either Eto or high-dose INH), an alternative agent, such as clofazimine or para-aminosalicylate, is considered for addition to the regimen to ensure a minimum of four effective drugs. Furthermore, if any of the four presumed effective drugs in the standard regimen are contraindicated or later withdrawn (owing to adverse events or poor tolerance), an alternative agent should be considered for substitution, provided it is not added in isolation to a failing regimen.
As 10% of MDR cases in SA are XDR,[2] initial treatment with standard MDR regimens may contribute to the development of amplified drug resistance owing to the selective pressure of treatment with only one or two effective drugs.[15] Until rapid second-line diagnostics are routinely available for immediate detection of pre-XDR and XDR, it is crucial that phenotypic second-line DST results are followed up after initiation of standard MDR treatment to allow regimens to be modified as soon as possible according to the full resistance pattern and to improve the individual's chance of cure. Furthermore, patients with MDR-TB, in whom standard treatment is failing (clinical deterioration, delayed sputum culture conversion, or reconversion to positive), the regimen should ideally be strengthened in consultation with an experienced clinician. Treatment options for individualised regimens in cases of pre-XDR, XDR-TB or MDR failure are severely limited and access to specific drugs is often, but not always, restricted to specialist hospital settings. With current XDR treatment success rates of <20%,[2] there is little point reserving drugs for salvage regimens at a later stage, and the most likely effective drugs should be offered as soon as possible.
There are a number of new TB drugs in the late phases of clinical development, such as bedaquiline (BDQ), delaminid and PA-824, and combinations of new and repurposed drugs (e.g. linezolid) are being compared with existing drugs in clinical trials to provide shorter, more effective and more tolerable regimens for DR-TB.[16] While awaiting the results of these trials, efforts are being made to increase access to new drugs for DR-TB patients with severely limited treatment options. The WHO issued interim guidance for use of BDQ for treating MDR-TB in June 2013 and the SA Medicines Control Council (MCC) has recently registered the drug for use outside of clinical trials. The MCC-approved national BDQ Clinical Access Programme has allowed access to the drug since 2013 - within optimised regimens for patients with pre-XDR and XDR-TB.[17] The experience gained from the programme will inform wider use of BDQ for other DR-TB patients once the drug is routinely available.
Summary
Case detection of DR-TB in SA has increased following nationwide roll-out of Xpert MTB/RIF. However, this may have limited impact on ongoing transmission of the disease unless the majority of cases rapidly initiate effective treatment. While an initial standardised treatment regimen may be more easily implemented at decentralised treatment initiation sites, the current MDR regimen should be optimised as soon as second-line susceptibility results are obtained to prevent acquisition of further resistance. Pre-XDR, XDR and MDR failure cases require early individualised treatment with all available, potentially effective drugs; however, treatment options remain severely limited. New and repurposed drugs may be made available for these cases through the national treatment programme within the framework of expert guidance while awaiting results of clinical trials for shorter, more effective and more tolerable regimens for all patients with DR-TB.
References
1. World Health Organization (WHO). Definitions and Reporting Framework for Tuberculosis - 2013 revision. Geneva: WHO, 2013. http://www.who.int/tb/publications/definitions/en/ (accessed 28 October 2014). [ Links ]
2. World Health Organization (WHO). Global Tuberculosis Report 2014. Geneva: WHO, 2014. http://www.who.int/tb/publications/global_report/en/ (accessed 28 October 2014). [ Links ]
3. World Health Organization (WHO). Companion Handbook to the WHO Guidelines for the Programmatic Management of Drug-resistant Tuberculosis. Geneva: WHO, 2014. http://www.who.int/tb/publications/pmdt_companionhandbook/en/ (accessed 28 October 2014). [ Links ]
4. Dheda K, Warren RM, Zumla A, Grobusch MP. Extensively drug-resistant tuberculosis: Epidemiology and management challenges. Infect Dis Clin North Am 2010;24(3):705-725. [http://dx.doi.org/10.1016/j.idc.2010.05.001] [ Links ]
5. South African Department of Health. Multi-drug Resistant Tuberculosis: A Policy Framework on Decentralised and Deinstitutionalised Management for South Africa. Pretoria: Department of Health, 2011. [ Links ]
6. Cox H, Hughes J, Daniels J, et al. Community-based treatment of drug-resistant tuberculosis in Khayelitsha, South Africa. Int J Tuberc Lung Dis 2014;18(4):441-448. [http://dx.doi.org/10.5588/ijtld.13.0742] [ Links ]
7. Lawn SD, Mwaba P, Bates M, et al. Tuberculosis 2013:1. Advances in tuberculosis diagnostics: The Xpert MTB/RIF assay and future prospects for a point-of-care test. Lancet Infect Dis 2013;13:349-361. http://dx.doi.org/10.1016/S1473-3099(13)70008-2] [ Links ]
8. South African Department of Health. National Tuberculosis Management Guidelines. Pretoria: Department of Health, 2014. [ Links ]
9. Rageade F, Picot N, Blanc-Michaud A, et al. Performance of solid and liquid culture media for the detection of Mycobacterium tuberculosis in clinical materials: Meta-analysis of recent studies. Eur J Clin Microbiol Infect Dis 2014;33:867-870. [http://dx.doi.org/10.1007/s10096-014-2105-z] [ Links ]
10. World Health Organization (WHO). Molecular Line Probe Assays for Rapid Screening of Patients at Risk of Multidrug-Resistant Tuberculosis (MDR-TB): Policy Statement. Geneva: WHO, 2008. [ Links ]
11. Morlock GP, Metchock B, Sikes D, et al. ethA, inhA, and katG loci of ethionamide-resistant clinical Mycobacterium tuberculosis isolates. Antimicrob Agents Chemother 2003;47(12):3799-3805. [http://dx.doi.org/10.1128/AAC.47.12.3799-3805.2003] [ Links ]
12. Schaaf HS, Victor TC, Engelke E, et al. Minimal inhibitory concentration of isoniazid in isoniazid-resistant Mycobacterium tuberculosis isolates from children. Eur J Clin Microbiol Infect Dis 2007;26(3):203-205. [http://dx.doi.org/10.1007/s10096-007-0257-9] [ Links ]
13. South African Department of Health. Management of Drug-Resistant Tuberculosis: Policy Guidelines. Pretoria: Department of Health, 2011. [ Links ]
14. Mphahlele M, Syre H, Valvatne H, et al. Pyrazinamide resistance among South African multidrug-resistant Mycobacterium tuberculosis isolates. J Clin Microbiol 2008;46(10):3459-3464. [http://dx.doi.org/10.1128/JCM.00973-08] [ Links ]
15. Müller B, Schaaf S, Gey van Pittius NC, et al. Current Standard Drug Regimens Facilitate the Evolution of Extensively Drug-Resistant Tuberculosis: Recommendations for Improvements. MRC Policy Brief. Parow: South African Medical Research Council, 2012. [ Links ]
16. RESIST-TB. DR-TB Clinical Trial Progress Report. http://www.resisttb.org/?page_id=1602 (accessed 28 October 2014). [ Links ]
17. Conradie F, Meintjes G, Hughes J, et al. Clinical access program for bedaquiline for the treatment of drug-resistant tuberculosis. S Afr Med J 2014;104(3):164. [http://dx.doi.org/10.7196/samj.7263] [ Links ]
 Correspondence:
Correspondence:
J Hughes
msfocb-khayelitsha-tbdoc@brussels.msf.org














