Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.104 n.12 Pretoria Dec. 2014
http://dx.doi.org/10.7196/SAMJ.8291
FORUM
CLINICAL ALERT
Tricuspid valve endocarditis associated with intravenous nyoape use: A report of 3 cases
R MeelI; F PetersII; M R EssopIII
IA cardiologist in the Division of Cardiology, Department of Internal Medicine, Chris Hani Baragwanath Academic Hospital and Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa. She is currently completing a PhD in valvular heart disease
IIA senior cardiologist in the Division of Cardiology and heads the echocardiography laboratory
IIIHead of the Division of Cardiology
ABSTRACT
We report three cases of tricuspid valve infective endocarditis associated with intravenous nyoape use. Nyoape is a variable drug combination of an antiretroviral (efavirenz or ritonavir), heroin, metamphetamines and cannabis. Its use is becoming increasingly common among poor communities in South Africa. All our patients were young HIV-positive men from disadvantaged backgrounds. They all presented with tricuspid regurgitation and septic pulmonary emboli. They were treated with prolonged intravenous antibiotic courses, and one required referral for surgery.
In developed countries, right-sided infective endocarditis (RSIE) frequently complicates intravenous drug use (IDU) and retroviral disease (HIV).[1]RSIE has been rare in sub-Saharan Africa in both the pre- and post-HIV eras, probably owing to the low frequency of IDU.[2,3] We report three cases of RSIE seen at a single hospital. All the patients were HIV-positive and were abusers of intravenous nyoape. The purpose of this report is to to alert the medical community to a new pattern of disease in at-risk populations. Failure to detect RSIE early may result in poor long-term outcomes.
Case summaries
Three young HIV-positive men were referred from peripheral hospitals to Chris Hani Baragwanath Academic Hospital (CHBAH), Soweto, Johannesburg, South Africa (SA). None of the patients was on highly active antiretroviral therapy. All admitted to intravenous nyoape abuse.
Case 1
The first patient was 29 years old and presented with a 1-week history of fever, dyspnoea and features suggestive of right heart failure and severe tricuspid regurgitation (TR). He had tachycardia (136 bpm), was tachypnoeic (respiratory rate 30/min) and had normal blood pressure. He had a raised jugular venous pressure, a soft first heart sound and a 4/6 pansystolic murmur, typically loudest over the epigastrium and accentuated by manoeuvres that increase venous return. The liver was enlarged and pulsatile. No peripheral stigmata of infective endocarditis were noted. A chest radiograph showed an increased cardiothoracic index with an opacified right costophrenic angle. An initial electrocardiogram (ECG) revealed only sinus tachycardia.
Inflammatory markers were elevated (white cell count (WCC) 18.7 x 109/L and C-reactive protein (CRP) 331 mg/L). The patient had a normochromic, normocytic anaemia (haemoglobin concentration 9.7 g/dL). The CD4+ count was 576 cells/μL. Blood cultures revealed Escherichia coli and salmonella (both only on a single set of cultures).
Transthoracic echocardiography (TTE) demonstrated severe TR (Fig. 1) secondary to a flail anterior tricuspid leaflet. The left-sided valves were normal. Large oscillating masses were noted on the leaflets (the largest measuring 25 mm), extending to the subvalvular apparatus and periannular area (Fig. 2). The pulmonary valve was normal. The right ventricle (RV) was mildly enlarged with normal systolic function but abnormal diastolic function with initially normal systolic pulmonary artery pressure and later pulmonary hypertension. The latter finding could be attributed to pulmonary emboli (Fig. 3). The later ECG showed new T-wave inversion in the anterior leads indicative of RV pressure overload with ischaemia.

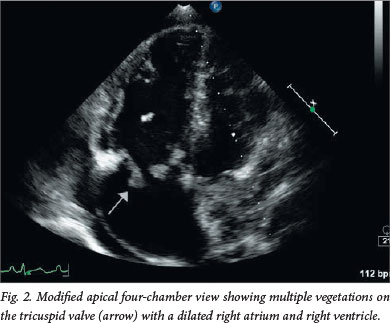

Definite tricuspid valve infective endocarditis (IE) was diagnosed based on the modified Duke criteria of one major criterion (oscillating masses on the valve leaflets) and three minor criteria (single positive blood cultures, IDU, fever (39°C) and suspected septic pulmonary emboli). A computed tomography pulmonary angiogram confirmed the presence of multiple bilateral pulmonary emboli complicated by infarction and cavitation.
The patient was commenced on treatment for IE, initially with gentamicin and cloxacillin. Five days later meropenem was substituted according to bacterial culture results. He also received diuretics. The inflammatory markers declined (CRP to 28 mg/L and the WCC to 10 x 109/L). The patient was referred for debridement and bioprosthetic tricuspid valve replacement because of severe TR, large vegetation size (>20 mm) and persistent pulmonary embolisation despite adequate antibiotic therapy.
Case 2
The second patient was 30 years old and presented with subacute, progressive dyspnoea as the primary complaint. Findings on clinical and special investigations were similar to the first case. A diagnosis of definite tricuspid valve IE was made based on the modified Duke criteria with one major (oscillating valve mass) and four minor criteria (IDU, suspected septic pulmonary infarcts, fever and a typical micro-organism (Staphylococcus aureus) on one blood culture). The patient continued to embolise to the lungs despite antibiotic therapy, and there was no improvement in the right heart failure. The patient had a history of defaulting from treatment, absconded from the hospital while on antibiotic therapy, and showed poor insight. He was treated conservatively.
Case 3
The third patient, 20 years old, had a 3-week history of fever and vague chest and right upper quadrant pain. A diagnosis of possible tricuspid valve IE was made based on the modified Duke criteria with one major (oscillating valve mass) and three minor criteria (IDU, fever, suspected septic pulmonary infarcts). No organisms were cultured owing to prior antibiotic administration. The patient was empirically treated with intravenous vancomycin and gentamicin.
The inflammatory markers improved, and he remained stable and was treated medically.
Discussion
To the best of our knowledge, these three cases are the first reported cases of RSIE secondary to intravenous nyoape use. Nyoape use is common in SA, especially among people from poor backgrounds.[4] Nyoape, also called whoonga or wunga, is a drug sold on the streets and has come to widespread use in SA since about 2010, initially among poor communities in Durban.[5] The ingredients are not exact or constant, but it usually contains an antiretroviral (ARV) such as efavirenz or ritonavir.[5] Other components include heroin, crystal methamphetamine, cannabis, cocaine and even rat poison.[5] Although nyoape was initially smoked, it is now being injected, as these cases suggest. This indicates a new pattern of drug use, which is likely to lead to an increase in cases of RSIE.
Heroin is the primary substance of abuse for 8% of individuals in treatment centres in Gauteng Province, SA. Nyoape is the most common form in which heroin is used by black South Africans, in particular, owing to its low cost. Users become addicted to the heroin component, but the neurological effects of efavirenz may play a role, as it is known to cause psychoactive symptoms.[5] ARVs may also potentiate the hypnotic effects of the other ingredients.[5] ARVs are sourced illegally from healthcare workers or are stolen from patients on ARVs.[5] This in turn may cause emergence of resistant HIV strains.[5]
IE affects right-sided valves in 5 - 10% of cases.[6] IE is most commonly seen in IDUs, especially in patients with concomitant HIV infection, as observed in our cases. The valve damage in RSIE is postulated to be secondary to poor hygiene in IDU, injection of contaminated matter or abnormalities of immune function.[6] The most common organism cultured is S. aureus, which accounts for 60 - 90% of cases.[6]S. aureus was cultured in only one of our patients, possibly because treatment with antibiotics had been instituted at the referring hospitals prior to a diagnosis being established.
All our patients were HIV-positive, and the following aspects specific to HIV and IE should be taken into consideration when managing these patients. Cardiac surgery is not contraindicated in patients with concurrent HIV and IE.[7] There is no increase in complications and mortality postoperatively.[7] In general, patients with HIV and IDU tend to have involvement of the left-sided valves more commonly than the right-sided valves.[8] In HIV-infected IDUs, the most common causative organism is still S. aureus.[9,10] In HIV-reactive (CD4+ count >350 cells/μL) IDUs, the odds ratio (OR) of developing IE is 2.31 compared with non-HIV-infected individuals. The OR increases to 8.31 at a CD4+ count of <350 cells/μL.[11] The mortality in patients with HIV and concomitant IE with a CD4+ count <200 cells/μL tends to be higher than in other groups.[12] More recent work, however, does not support the higher mortality in patients with low CD4+ counts.[10] There is no significant difference in terms of mortality and response to treatment with antimicrobials between HIV-infected and non-HIV-infected IDUs.[8,9,13,14]
A high level of suspicion needs to be maintained, as RSIE may present with nonspecific symptoms and lacks the usual peripheral stigmata associated with left-sided IE.[15] This results in delayed diagnosis and therapy of IE, as evident in all three of our cases. These cases highlight the importance of considering IE in a patient presenting with features suggestive of pneumonia coupled with right heart failure. Blood for culture (ideally three sets of cultures) should be drawn immediately in this context.
The more common complications of RSIE include fever and respiratory symptoms secondary to septic pulmonary emboli, as in our patients. Right-sided heart failure is uncommon. When it occurs, it is usually secondary to volume overload due to severe, organic TR or to pressure overload due to pulmonary hypertension caused by septic pulmonary emboli (or both).[6]
The diagnosis of IE on the tricuspid valve is made with TTE, which allows good visualisation of the valve because of its anterior location.[6] The overall sensitivity of TTE is 60 - 70%, but sensitivity increases to 80% in detection of right-sided endocarditis.[16] In most cases of RSIE, TTE is therefore sufficient. Transoesophageal echocardiography (TEE) is useful when the image quality is poor with TTE, TTE is negative in the presence of high clinical suspicion, or S. aureus septicaemia or a complication of IE is suspected.[6] The modified Dukes criteria are useful for the diagnosis of IE[6] but do not replace clinical judgement in cases where its sensitivity is reduced, such as in RSIE, especially in IDUs.[6]
The management of RSIE is largely conservative, with most cases treated medically. Empirical antibiotic treatment must cover S. aureus, for which vancomycin would be the drug of choice. The choice of empirical treatment will also be dictated by the suspected organism, IDU, and the valve involved.[6] Duration of antibiotic therapy ranges from 2 weeks in certain uncomplicated cases to up to 6 weeks in complicated cases.[6]
Surgery is only considered in patients with intractable right-sided heart failure, unresponsive to heart failure treatment in the form of diuretics; difficult-to-eliminate organisms; persistent bacteraemia (minimum of 1 week's duration), despite appropriate antibiotics; and large tricuspid valve vegetations of >2 cm that persist and are complicated by recurrent embolisation to the lung, in the presence or absence of right heart failure.[6] Our first patient was referred for surgery because of recurrent pulmonary emboli with worsening pulmonary hypertension, despite being on appropriate antibiotic therapy.
The goal of surgery in RSIE is to achieve complete debridement of the infected tissue, and preferably valve repair. If repair is not feasible owing to extensive valve destruction, valve replacement with a bioprosthetic valve should be undertaken. A simple valvectomy, as advocated traditionally, may result in worsening of right-sided haemodynamics postoperatively, if pulmonary hypertension has been present preoperatively.[6]
Lastly, the second patient with poor insight constitutes an ethical dilemma regarding conservative v. surgical treatment, especially in a resource-limited setting. From the limited available literature, surgery should be offered for the first episode of IE in IDUs who are willing to undergo rehabilitation. If the patient presents with a second episode of IE secondary to lack of compliance, he or she should probably not qualify for further surgical intervention.[17]
Since the time of writing, an additional three cases with a similar presentation have been seen.
Conclusion
We are likely to encounter more cases of RSIE secondary to intravenous nyoape use with concurrent HIV infection. A high level of vigilance should be maintained and diagnosis made early before complications arise.
References
1. Akinosoglou K, Apostolakis E, Marangos M, et al. Native valve right sided infective endocarditis. Eur J Intern Med 2013;24(6):510-519. [ Links ]
2. Naidoo DP. Right-sided endocarditis in the non-drug addict. Postgrad Med J 1993;69(814):615-620. [ Links ]
3. Ndiaye MB, Diao M, Pessinaba S, et al. Epidemiological, clinical and ultrasonographic aspects of right-sided infective endocarditis in Senegal: 6 cases. Med Trop (Mars) 2011;71(5):484-486. [ Links ]
4. South African Community Epidemiology Network on Drug Use report. www.sahealthinfo.org/admodule/sacendu/Sacenduphase34.pdf (accessed 9 January 2014). [ Links ]
5. Whoonga. Wikipedia encyclopedia. http://en.wikipedia.org/wiki/Whoonga (accessed 9 January 2014). [ Links ]
6. Habib G, Hoen B, Tornos P, et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis. Eur Heart J 2009;30(19):2369-2413. [http://dx.doi.org/10.1093/eurheartj/ehp285] [ Links ]
7. Mestres C-A, Chuqoiure JE, Claramonte X, et al. Long term results after cardiac surgery in patients infected with the human immunodeficiency virus type-1 (HIV). Eur J Cardiothorac Surg 2003;23(6):1007-1016. [ Links ]
8. Nahass R, Weinstein MP, Bartels J, et al. Infective endocarditis in intravenous drug user: A comparison of human immunodeficiency virus type1-negative and -positive patients. J Infect Dis 1990;162(4):967-970. [http://dx.doi.org/10.1093/infdis/162.4.967] [ Links ]
9. Miro JM, del Rio A, Mestres CA. Infective endocarditis in intravenous drug abusers and HIV-1 infected patients. Infect Dis Clin North Am 2002;16(2):273-295. [ Links ]
10. Fernández Guerrero M, González López JJ, Goyenechea A, et al. Endocarditis caused by Staphylococcus aureus: A reappraisal of the epidemiologic, clinical, and pathologic manifestations with analysis of factors determining outcome. Medicine (Baltimore) 2009;88(1):1-22. [http://dx.doi.org/10.1097/MD.0b013e318194da65] [ Links ]
11. Manoff S, Vlahov D, Herskowitz A, et al. Human immunodeficiency virus and infective endocarditis among injecting drug users. Epidemiology 1996;7(6):566-570. [ Links ]
12. Ribera E, Miro JM. Influence of HIV 1 infection and degree of immunosuppression in the clinical characteristics and outcome of infective endocarditis in intravenous drug users. Arch Intern Med 1998;158(18):2043-2050. [http://dx.doi.org/10.1001/archinte.158.18.2043] [ Links ]
13. Valencia E, Miro J. Endocarditis in the setting of HIV infection. AIDS Rev 2004;6(2):97-106. [ Links ]
14. Miro JM, del Rio A, Mestres CA. Infective endocarditis and cardiac surgery in intravenous drug abusers and HIV-1 infected patients. Cardiol Clin 2003;21(2):167-184. [ Links ]
15. Fernández Guerrero ML, Alvarez B, Manzarbeitia F, Renedo G. Infective endocarditis at autopsy: A review of pathologic manifestations and clinical correlates. Medicine (Baltimore) 2012;91(3):152-164. [http://dx.doi.org/10.1097/MD.0b013e31825631ea] [ Links ]
16. San Roman JA, Vilacosta I, Zamorano JL, Almeria C, et al. Transesophageal echocardiography in right-sided endocarditis. J Am Coll Cardiol 1993;21(5):1226-1230. [http://dx.doi.org/10.1016/0735-1097(93)90250-5] [ Links ]
17. Yeo KK, Chang WS, Lau JM, et al. Valve replacement in endocarditis: Setting limits in noncompliant intravenous drug abusers. Hawaii Med J 2006;65(6):168-171. [ Links ]
 Correspondence:
Correspondence:
R Meel
ruchikameel@gmail.com
Accepted 4 June 2014.














