Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.104 n.11 Pretoria Nov. 2014
http://dx.doi.org/10.7196/SAMJ.8619
REVIEW
PAEDIATRIC HEPATOBILIARY
The role of interventional radiology in complications after paediatric liver transplantation
J Cantrell
MB BCh, FCRad (SA); Wits Donald Gordon Medical Centre, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
Liver transplantation has become an established treatment in both adults and children for end-stage liver disease, acute hepatic failure and certain liver tumours. There is a significant risk of complications after all forms of liver transplantation. The interventional radiologist plays a critical role in the diagnosis and treatment of these complications. The use of image-guided, minimally invasive procedures reduces the need for surgical revision or retransplantation and improves graft and patient survival rate. This article reviews some of the most common vascular and non-vascular complications after paediatric liver transplantation, and the interventional radiology techniques used to diagnose and treat them.
Originally, all liver transplants used deceased donors. There have been major advances over the last few decades, with the introduction of split liver grafts and living related donor transplants dramatically increasing the pool of potential donors. Ongoing technical advances have improved the rate of both graft and patient survival, but a liver transplant remains a procedure with significant morbidity and mortality.1 A multidisciplinary approach to the post-transplant patient is vital, and image-guided, minimally invasive procedures improve graft and patient survival.2
Image-guided liver biopsy
Liver biopsy is a common procedure that is performed if there is unexplained derangement of liver functions, such as seen with rejection, ischaemia or cholestasis without biliary dilatation.3 Most biopsies are performed percutaneously, unless the patient has a severe coagulopathy, in which case a transjugular biopsy is performed. Percutaneous biopsies are done with anaesthesia and under ultrasound (US) guidance, either using a right anterolateral or an epigastric approach, depending on the position of the graft. At our institution we use a Menghini-type suction needle to obtain core samples of the liver for histology. There is a risk of perihepatic haematoma, so compression is manually applied over the biopsy site for a few minutes post procedure. If there is ascites overlying the liver, this is first drained percutaneously.
Biliary complications
The rate of biliary complication is between 11% and 25%, and complications are more common in reduced, split and living related donor grafts, compared with whole grafts. Most biliary complications can be treated with interventional radiology (IR) techniques alone.4 Biliary complications include bile duct stenosis, bile leaks and intraparenchymal or perihepatic biloma. The majority of complications occur within 3 months post transplant.
Biliary stenosis
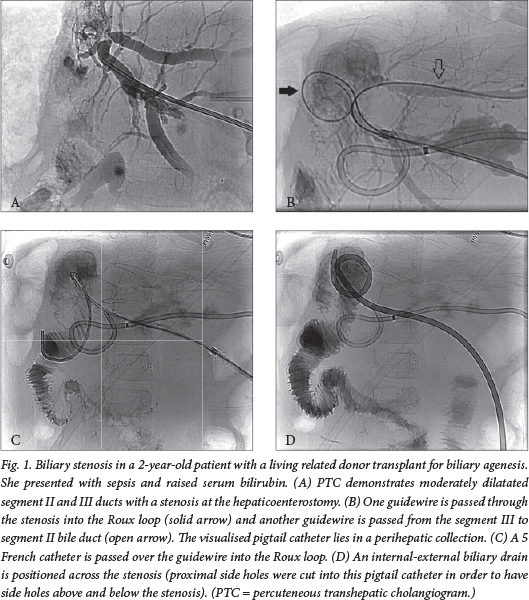
Biliary stenosis can occur at the anastomosis, usually caused by scarring, or less commonly at a non-anastomotic site, usually caused by ischaemia, infection or rejection. A stenosis is suspected if US, computerised tomography (CT) or magnetic resonance imaging demonstrates dilatated bile ducts upstream of the stenosis, but a significant number of obstructed bile ducts appear normal on US; percutaneous transhepatic cholangiography (PTC) is often required for a definitive diagnosis.5 Most transplants use a Roux loop and hepaticoenterostomy, precluding the use of endoscopic retrograde cholangiopancreatography to image or treat the biliary tree.
When a PTC confirms a stricture, balloon dilatation is performed and an internal-external biliary drain is placed across the stricture to prevent restenosis (Fig. 1). The biliary drain is left in situ for 4 - 6 weeks and dilatation is usually repeated on a few occasions until the cholangiogram demonstrates resolution of the stricture with adequate bile drainage, at which point the biliary drain is removed. Self-expanding metal stents are reserved for the few cases resistant to repeat balloon dilatation.
Bile leak
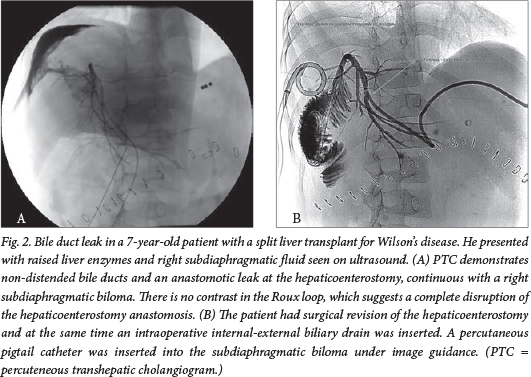
Bile leaks are well demonstrated with PTC and most often occur at the anastomosis. They are usually treatable with an internal-external biliary drain that crosses the anastomosis and is left in situ for a few weeks. The drain can be removed once cholangiography, through the drain access, shows no further leakage. If there is complete disruption of the anastomosis, surgical revision is necessary (Fig. 2). Non-anastomotic bile leaks can occur on the cut surface of the liver in the case of split and living related donor transplants.
Biloma
Perihepatic bilomas are common and occur if there is a bile duct leak; small bilomas can be treated conservatively. It is also common to have a bile collection adjacent to the cut edge of the liver in the case of a split liver transplant, which may on occasion be associated with stenosis at the hepaticojejunal anastomosis. Bilomas increase the risk of sepsis and can be treated by means of a percutaneous pigtail catheter, usually inserted under US and fluoroscopic guidance.
Vascular complications
Hepatic artery stenosis
The rate of hepatic artery stenosis (HAS) is between 11% and 19%6 and stenosis usually occurs within 3 months post transplant. HAS increases the risk of ischaemia and hepatic artery thrombosis (HAT). In adults, 65% of untreated HAS progresses to thrombosis within the first 6 months.7 Stenoses usually occur at the site of anastomosis but can occur elsewhere as a result of rejection and ischaemia. There is often a nonspecific clinical picture, which usually includes abnormal liver function tests, and diagnosis is usually made on Doppler US. Stenosis is suspected if there is spectral broadening and an increase in the peak velocity above 2 m/s at the site of the anastomosis. Sometimes the stenosis itself is obscured on US, but if there is an abnormal waveform in the distal artery, with an acceleration time of >0.08 seconds and a resistive index <0.5, it indicates the presence of a stenosis.
If HAS is suspected, a formal angiogram can confirm the stricture, and the trans-stenotic pressure is measured to confirm a pressure gradient (Fig. 3). A gradient above 10 mmHg is considered significant. A microcatheter is necessary owing to the small calibre of the hepatic artery.7
Long-term patency rates after percutaneous transluminal angioplasty (PTA) in adults are 60 - 80% at 1 year, but there are poor data on long-term studies in children. PTA is typically performed with a 2 - 5 mm balloon; stents are reserved for when repeat PTAs are unsuccessful. Complications such as rupture or dissection can also be treated with stents.3
Hepatic artery thrombosis
HAT is an infrequent but devastating post-transplant complication with high morbidity and mortality, often leading to graft loss if untreated. The reported incidence of HAT is highly variable, but is ~5% in high-performing transplant centres.8 Doppler US is sensitive in detecting HAT (92%), with non-visualisation of the artery and loss of arterial Doppler signal along the anticipated course of the hepatic artery.9 Surgical treatment options include thrombectomy with revascularisation (if diagnosed early) and repeat transplantation. Endovascular thrombolytic treatment (selective percutaneous thrombolysis followed by angioplasty) has been described in paediatric transplant HAT, but there are few data on long-term studies in children.10
Portal vein stenosis
The rate of portal vein complications, including portal vein stenosis (PVS), is between 4% and 8%.11 Patients typically present with signs of portal hypertension such as splenomegaly, variceal bleeding and ascites. Diagnosis is usually made with Doppler US. The stenosis is best confirmed with direct portal venography via transhepatic puncture, where the pressure gradient across the stricture can also be measured. An indirect portogram (on the delayed phase of a superior mesenteric angiogram) also allows visualisation of the portal vein, but no direct access for interventions.
Balloon angioplasty (Fig. 4) results in good long-term patency rates, with 10-year primary patency rates of 70% and 10-year primary assisted patency rates (patency of the stenosis after repeat intervention following symptomatic restenosis at the same site) of 96%. Metal stents are reserved for recurrent or elastic lesions.12

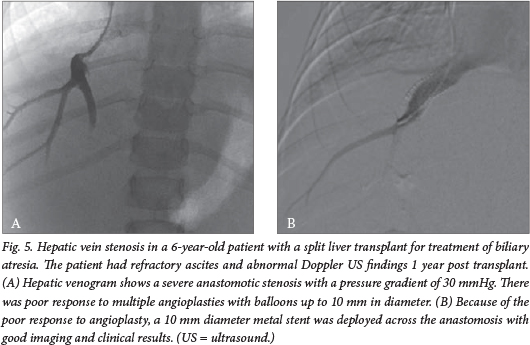
Hepatic vein stenosis
Hepatic venous outflow obstruction is an uncommon complication, with an overall incidence of 6%. Patients typically present with ascites, abnormal liver function tests and new-onset splenomegaly.
Percutaneous balloon angioplasty is a very effective method of treating hepatic venous outflow obstruction, with 10-year primary patency rates of 52% and 10-year primary assisted patency rates of 95%.13
A venogram is performed and the pressure gradient is measured to confirm the diagnosis, usually via a transjugular or transfemoral route (Fig. 5). After balloon dilatation, the stenosis may recur, requiring reintervention. Insertion of a metal stent is reserved for cases that do not respond to repeat balloon dilatation. Doppler US is used for short- and long-term follow-up.
Inferior vena cava stenosis
Stenosis of the inferior vena cava is uncommon, with an incidence of <2%. The stenosis is usually at the superior anastomosis and can occur in conjunction with a hepatic vein stenosis. Patients present with hepatomegaly, ascites, lower limb oedema, pleural effusions or abnormal liver function tests. The diagnosis is usually made with Doppler US, which can demonstrate the stenosis, sometimes with adjacent thrombus and associated increased flow velocities.
A cavogram confirms the stenosis, and the trans-stenotic pressure gradient is measured before and after angioplasty. As with most venous stenoses, balloon angioplasty is the treatment of choice, with stent placement reserved for post-dilatation pressure gradients of >5 mmHg or persistent stenosis on post-angioplasty cavogram.14
Aspiration of pleural effusions
Pleural effusions are common post transplant, occurring in 76% of patients, and most occur on the right. Approximately 40% of these effusions will persist for >7 days, and this is associated with an increased incidence of graft rejection.15 When there are signs of sepsis or the effusion is large, the effusion is aspirated under US guidance and the fluid is sent for a microscopy, culture and sensitivity test. When there is rapid reaccumulation of pleural fluid or the fluid is clearly infected, a percutaneous pigtail catheter may be inserted and allowed to drain into a catheter bag.
Percutaneous drainage of collections
Post-transplant collections are common and can include postoperative perihepatic haematomas, bilomas and ascites. These collections are at risk of becoming septic, and often have a nonspecific presentation; a high index of suspicion for infection must be maintained.
Collections, haematomas and ascites are identified on US or CT, and vary from simple to gelatinous in consistency. If there is suspicion of infection or a significant mass effect, collections can be aspirated or a percutaneous pigtail catheter can be inserted. Both are usually done using a combination of US and fluoroscopic guidance, or with CT guidance alone.
Experience at the Wits Donald Gordon Medical Centre (WDGMC)
Since 2005,60 paediatric liver transplants have been performed in 58 patients at the WDGMC. Only one patient received radiological vascular intervention, this being a PVS treated with angioplasty, later requiring re-angioplasty and placement of a metal stent.
In the 3-year period between April 2011 and April 2014, 25 transplants were performed on 24 patients, and 82 IR procedures were performed on these patients (an average of 3.3 IR procedures per transplant).
Five of the 24 patients (21%) had no IR procedures, and 19 of the 24 patients (79%) had one or more IR procedures (Table 1)

Conclusion
Liver transplantation is established as a very successful treatment of end-stage liver disease and several other metabolic diseases in children, but vascular and non-vascular complications may occur. An array of image-guided, minimally invasive techniques allow an interventional radiologist to treat many of these complications, improving graft and patient survival and reducing the rate of surgical revision and retransplantation.
Acknowledgements. Case courtesy of Dr C Sanyika: Fig. 2. Cases courtesy of Miraglia R, Maruzzelli L, Caruso S, et al. Interventional radiology procedures in pediatric patients with complications after liver transplantation. Radiographics 2009;29(2):567-584: Figs 3, 4 and 5.
REFERENCES
1. Spada M, Riva S, Maggiore G, et al. Pediatric liver transplantation. World J Gastroenterol 2009;15(6):648. [ Links ]
2. Denys A, Chevallier P, Doenz F, et al. Interventional radiology in the management of complications after liver transplantation. Eur Radiol 2004;14(3):431-439. [http://dx.doi.org/10.1007/s00330-003-2196-x] [ Links ]
3. Miraglia R, Maruzzelli L, Caruso S, et al. Interventional radiology procedures in pediatric patients with complications after liver transplantation. Radiographics 2009;29(2):567-584. [http://dx.doi.org/10.1148/rg.292085037] [ Links ]
4. Heffron TG, Pillen T, Welch D, et al. Biliary complications after pediatric liver transplantation revisited. Transplant Proc 2003;35(4):1461-1462. [ Links ]
5. Kok T, Van der Sluis A, Klein JP, et al. Ultrasound and cholangiography for the diagnosis of biliary complications after orthotopic liver transplantation: A comparative study. J Clin Ultrasound 1996;24(3):103-115. [ Links ]
6. Moray G, Boyvat F, Sevmiş S, et al. Vascular complications after liver transplantation in pediatric patients. Transplant Proc 2005;37(7):3200-3202. [http://dx.doi.org/10.1016/j.transproceed.2005.08.045] [ Links ]
7. Saad WE, Davies MG, Sahler L, et al. Hepatic artery stenosis in liver transplant recipients: Primary treatment with percutaneous transluminal angioplasty. J Vasc Interv Radiol 2005;16(6):795-805. [http://dx.doi.org/10.1097/01.RVI.0000156441.12230.13] [ Links ]
8. Englesbe MJ, Kelly B, Goss J, et al. Reducing pediatric liver transplant complications: A potential roadmap for transplant quality improvement initiatives within North America. Am J Transplant 2012;12(9):2301-2306. [http://dx.doi.org/10.1111/j.1600-6143.2012.04204.x] [ Links ]
9. Flint EW, Sumkin JH, Zajko AB, et al. Duplex sonography of hepatic artery thrombosis after liver transplantation. AJR Am J Roentgenol 1988;151(3):481-483. [http://dx.doi.org/10.2214/ajr.151.3.481] [ Links ]
10. López-Benítez R, Schlieter M, Hallscheidt PJ, et al. Successful arterial thrombolysis and percutaneous transluminal angioplasty for early hepatic artery thrombosis after split liver transplantation in a four-month-old baby. Pediatr Transplant 2008;12(5):606-610. [http://dx.doi.org/10.1111/j.1399-3046.2008.00925.x] [ Links ]
11. Yabuta M, Shibata T, Shibata T, et al. Long-term outcome of percutaneous transhepatic balloon angioplasty for portal vein stenosis after pediatric living donor liver transplantation: A single institute's experience. J Vasc Interv Radiol 2014;25(9):1406-1412. [http://dx.doi.org/10.1016/j.jvir.2014.03.034] [ Links ]
12. Funaki B, Rosenblum JD, Leef JA, et al. Portal vein stenosis in children with segmental liver transplants: Treatment with percutaneous transhepatic venoplasty. AJR Am J Roentgenol 1995;165(1):161-165. [http://dx.doi.org/10.2214/ajr.165.1.7785578] [ Links ]
13. Yabuta M, Shibata T, Shibata T, et al. Long-term outcome of percutaneous interventions for hepatic venous outflow obstruction after pediatric living donor liver transplantation: Experience from a single institute. J Vasc Interv Radiol 2013;24(11):1673-1681. [http://dx.doi.org/10.1016/j.jvir.2013.07.010] [ Links ]
14. Lorenz J, Van Ha T, Funaki B, et al. Percutaneous treatment of venous outflow obstruction in pediatric liver transplants. J Vasc Interv Radiol 2006;17(11):1753-1761. [http://dx.doi.org/10.1097/01.RVI.0000241540.31081.52] [ Links ]
15. Mack CL, Millis JM, Whitington PF, et al. Pulmonary complications following liver transplantation in pediatric patients. Pediatr Transplant 2000;4(1):39-44. [http://dx.doi.org/10.1034/j.1399-3046.2000.00080.x] [ Links ]
 Correspondence:
Correspondence:
J Cantrell
Cantrell_j@hotmail.com














