Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SAMJ: South African Medical Journal
versión On-line ISSN 2078-5135
versión impresa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.104 no.11 Pretoria nov. 2014
http://dx.doi.org/10.7196/SAMJ.8959
ARTICLES
CONTINUING MEDICAL EDUCATION
Advances in the diagnosis and management of allergic disease: applications to South African practice
A PentzI; R J GreenII
IMB ChB, DCH (SA), FCPaed (SA), MMed (Paed), Dip Allergology (SA), Cert Pulmonolgy (Paed) (SA), FCCP;Department of Paediatrics and Child Health, Faculty of Health Sciences, University of Pretoria, South Africa
IIMB BCh, DCH, FCPaed (SA), DTM&H, MMed, FCCP, PhD, Dip Allergology (SA), FAAAAI, FRCP, DSc; Department of Paediatrics and Child Health, Faculty of Health Sciences, University of Pretoria, South Africa
ABSTRACT
There have been a number of advances in the diagnosis and management of allergic diseases that are relevant to South African (SA) circumstances. These are all published or about to be published in new guidelines that provide practical advice to guide SA doctors who treat patients with these conditions. The guidelines include those for atopic dermatitis, allergic rhinitis and food allergy. This article reflects the most pertinent aspects of the guidelines. It also provides a short summary of a new allergy diagnostic test available in SA, the multiplex microarray chip, known as the immuno-solid-phase allergen chip (ISAC) test. It provides component-resolved allergy testing for special circumstances and complex allergic problems and is certainly not required as a screening allergy test. Finally, this article gives an update on allergen immunotherapy - some patients with allergic conditions may benefit from immunotherapy. In SA, some forms of immunotherapy for allergic rhinitis and mild asthma may currently include sublingual immunotherapy.
The prevalence of allergic diseases is rising worldwide and certainly in South Africa (SA). Every few weeks the major allergy journals list new allergens identified as triggering allergic reactions. There have recently been significant advances in the diagnosis and management of allergic disorders.
It may be prudent to consider diagnostic and management advances for the SA generalist in two ways. There are a host of new SA guidelines for the diagnosis and management of all the allergic diseases; these might be considered as the 'practical advances'. There are also new diagnostic and therapeutic approaches that may be considered as the 'possible advances'. 'Possible', because even though these advanced tests and therapies are available they should not be utilised without proper cause.
This article focuses on these two approaches, the latest guideline suggestions and some of the new ideas regarding diagnosis in the laboratory and therapy in the clinic.
Practical advances
A number of SA 'guidelines' have been published in the past year and should form the basis of any rational decision-making in the diagnosis and management of allergic disorders.
The general approach to the management of atopic dermatitis can be presented schematically (Fig. 1).1
The SA Allergic Rhinitis Working Group published the requirements with regard to the management of chronic rhinitis (CR) (Table 1).2
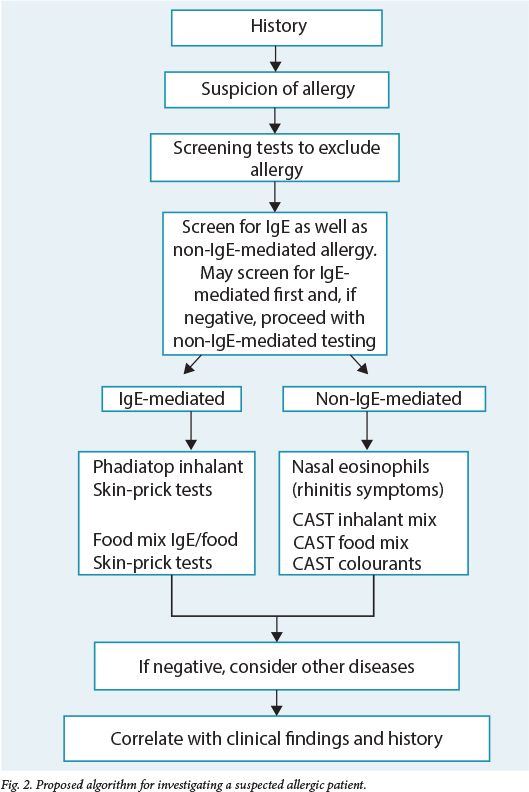
The Allergy Society of SA, together with the National Pathology Group, revised algorithms for the investigation of allergic patients. The algorithm for investigating a suspected allergic patient summarises that approach (Fig. 2).3
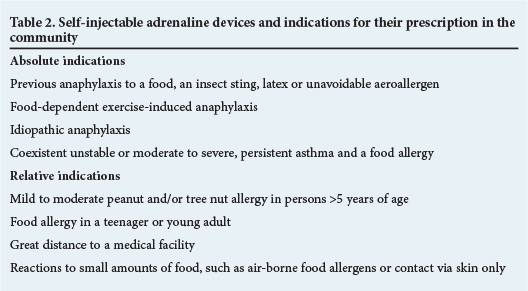
In late 2013 the Allergy Society of SA, in partnership with other stakeholders, formulated food allergy guidelines for SA. In those guidelines there is a statement on indications for the prescription of adrenaline (EpiPen) to patients (Table 2).4 The same document listed the prevalence and prognosis of various food allergies (Table 3). These are international figures, whereas SA data are still being accumulated.

Possible advances
There have been a number of advances in allergy diagnostics and therapy. Two of these are the development of a new diagnostic modality, the multiplex microarray chip, and in the therapeutic realm new progress in immunotherapy.
Immuno-solid-phase allergen chip (ISAC) microarray test[5]
The ISAC is a multiplex microarray chip test in which immunoglobulin (Ig) E is detected to multiple recombinant allergen components. The current ISAC microchip is a miniaturised immunoassay platform using only 20 µl of serum to measure specific IgE to 112 different recombinant allergen components. It should not be used as a screening test in patients with a history of a low suspicion of allergy, but should be used as a diagnostic tool in patients with suspected allergen cross-reactivity such as combined food and pollen allergies or in patients with multiple allergies.
Clinical value of IgE testing to allergen components
One of the major advantages of component testing is the ability to distinguish between primary, species-specific sensitisation, and cross-reactivity to proteins with similar protein structures, which may contribute towards evaluating the risk of reaction on exposure to different allergens. Protein structure and stability to heat and digestion may affect tolerance to raw or cooked foods and the severity of clinical reactions. This information can be used to individualise patient management by including advice on targeted allergen exposure reduction, selection of suitable allergens for specific immunotherapy or need to perform food challenges.6-9
Pollen allergy and cross-reactive food allergy
Patients with pollen allergies are often sensitised to cross-reactive components that occur in pollens and foods of plant origin. The most common ones are cross-reactive carbohydrate determinants (CCD), profilins, proteinase-10 (PR-10) and lipid transfer proteins (LTP). Clinical relevance and severity of reactions can be predicted, e.g. IgE to CCD is the least likely to cause symptoms. The likelihood of symptomatic allergy increases with IgE to profilin, then PR-10 and LTP, the last being the most likely to induce clinically relevant reactions. Profilin and PR-10 are also heat labile; therefore patients with this allergy may be able to tolerate cooked food while being symptomatic to raw food. Knowledge of protein localisation may also contribute to patient management, as PR-10 is mainly localised to the pulp of fruit and LTP to the peel. Patients with LTP allergy may sometimes be able to tolerate peeled fruit. As cross-reactivity may have a substantial impact on patient management, there should be a greater awareness when interpreting allergy tests regarding potential cross-reactivity between pollens and food of plant origin. A typical pointer to potential cross-reactivity is pollens and several foods of plant origin that are positive on IgE-mediated allergy tests, especially positive reactions to a combination of wheat, peanut and soy.
Milk allergy
The major allergens are casein (Bos d 8), alpha-lactalbumin (Bos d 4) and beta-lactoglobin (Bos d 5), although allergies to minor proteins such as bovine serum albumin (BSA) (Bos d 6) and lactoferrin (Bos d lactoferrin) have been reported. Casein, the most important and abundant allergen in milk and hard cheese, is heat stable. Patients with high levels of IgE to casein are at risk for severe reactions and are less likely to outgrow their milk allergy. Note that there is a high homology between casein of different species, and patients with casein reactivity have a high risk of reacting to the milk of other animal species. Whey proteins (alpha-lactalbumin and beta-lactoglobin) are heat labile and patients reacting to these proteins may often tolerate heated or fermented milk products. BSA is a serum albumin that is a main protein in mammalian blood and an important allergen involved in milk, meat and epithelia allergy. Sensitised patients may react to different meats (beef, lamb and pork), epithelia (cat and dog) and cow's milk.
Egg allergy
Egg white is the most important source of egg allergy and contains 23 different proteins. The most important allergens are ovomucoid (Gal d 1), ovalbumin (Gal d 2), ovotransferrin/conalbumin (Gal d 3) and lysozyme (Gal d 4). Although ovomucoid comprises only 10% of the total egg white protein, it has been shown to be the dominant allergen and is allergenic in minute amounts. This protein is very stable to heat and digestion; therefore, allergic patients cannot tolerate egg in baked products. High levels of IgE to Gal d 1 are also associated with persistent egg allergy. However, absence or low levels of IgE antibodies to Gal d 1 are associated with an increased probability of tolerance to ingestion of cooked egg. This may guide clinicians in when they should perform a cooked egg challenge. Gal d 5 is present as the protein egg livetin in egg yolk and in chicken as chicken serum albumin and may cause 'bird-egg syndrome', where patients may react to egg yolk, chicken meat and feathers.
Immunotherapy
Allergen immunotherapy has been used to treat allergic diseases, such as asthma, allergic rhinitis, and venom allergy for more than a century. Subcutaneous immunotherapy involves the administration of clinically relevant allergens for several months, building up to eventual monthly injections - typically for 3 - 5 years.
Recent advances have improved the safety and efficacy of immunotherapy. The addition of omalizumab or toll-like receptor agonists to standard subcutaneous immunotherapy has proved beneficial. Altering the extract itself, either through chemical manipulation producing allergoids or directly producing recombinant proteins or significant peptides, has been evaluated - with promising results. The use of different administration techniques, such as sublingual immunotherapy, is common in SA. Other methods of administering allergen immunotherapy have been studied, including epicutaneous, intralymphatic, intranasal, and oral immunotherapy.10
For patients with certain allergic profiles, immunotherapy may be an important treatment modality. Such patients include those with bee venom anaphylaxis, allergic rhinitis and mild asthma.
New in this arena is that immunotherapy no longer requires receiving injections for years. For aeroallergen allergy oral and sublingual immunotherapy is now available. The indications for immunotherapy are listed in Table 4.11

Conclusion
This article summarises the important facts present in SA guidelines, which summarise current trends in the management of common allergic diseases and have locally relevant practice suggestions.
Acknowledgement. The authors would like to thank Dr Cathy van Rooyen for permission to use the extract on the 'ISAC microarray test'.5
REFERENCES
1. Green RJ, Sinclair W. General approach to and summary of the guideline for the management of atopic dermatitis. S Afr Med J 2014;104(10). [http://dx.doi.org/10.7196/SAMJ.8876] [ Links ]
2. Green RJ, Hockman M, Friedman R, et al. Chronic rhinitis in South Africa: Update 2103. S Afr Med J 2013;103:419-422. [http://dx.doi.org/10.7196/SAMJ.6972] [ Links ]
3. National Pathology Group Working Party. Guideline for diagnostic testing in allergy. http://www.pathology.co.za (accessed 1 October 2014). [ Links ]
4. Levin ME, Gray CL, Goddard E, et al. South African food allergy consensus 2015. S Afr Med J (in press). [ Links ]
5. Van Rooyen C. Advances in allergy diagnostics. Current Allergy & Clinical Immunology 2014;27(1):20-26. [ Links ]
6. Sastre J. Molecular diagnosis and immunotherapy. Curr Opin Allergy Clin Immunol 2013;13(6):646-650. [http://dx.doi.org/10.1097/ACI.0b013e328364f4c6] [ Links ]
7. Harwanegg C, Hiller R. Protein microarrays in diagnosing IgE-mediated diseases: Spotting allergy at the molecular level. Expert Rev Mol Diagn 2004;4(4):89-98. [http://dx.doi.org/10.1586/14737159.4.4.539] [ Links ]
8. Borres MP, Ebisawa M, Eigenmann PA. Use of allergen components begins a new era in paediatric allergology. Pediatr Allergy Immunol 2011;22:454-461. [http://dx.doi.org/10.1111/j.1399-3038.2011.01197.x] [ Links ]
9. Sastre J, Landivar ME, Ruiz-Garcia M, Adregnette-Rosigno MV, Mahillo I. How molecular diagnosis can change allergen-specific immunotherapy prescription in a complex pollen area. Allergy 2012;67(5):709-711. [http://dx.doi.org/10.1111/j.1398-9995.2012.02808.x] [ Links ]
10. Casale TB, Stokes JR. Immunotherapy: What lies beyond. J Allergy Clin Immunol 2014;133(3):612-619. [http://dx.doi.org/10.1016/j.jaci.2014.01.007] [ Links ]
11. Potter P, Weinberg EG. Allergen immunotherapy. In: Green RJ, Motala C, Potter P, eds. ALLSA Handbook of Practical Allergy. Cape Town: ALLSA, 2010. [ Links ]
12. South African Department of Health. Standard Treatment Guidelines and Essential Medicine List. Pretoria: Department of Health, 2013. http://www.health.gov.za/edp.php (accessed 9 October 2014). [ Links ]
 Correspondence:
Correspondence:
R J Green
robin.green@up.ac.za














