Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.104 n.11 Pretoria Nov. 2014
http://dx.doi.org/10.7196/SAMJ.8518
FORUM
CLINICAL ALERT
Blood-borne infections in healthcare workers in South Africa
T M RossouwI; M van RooyenII; J M LouwIII; K L RichterIV
IDr Theresa Rossouw is an HIV clinician and researcher in the departments of Family Medicine and Immunology at the University of Pretoria, South Africa. She has been involved in the management of HIV-infected patients and students since 2004 and has a special interest in drug-resistant HIV and biomedical ethics.
IIDr Marietjie van Rooyen is a family physician working with both undergraduate and postgraduate students in the Department of Family Medicine, University of Pretoria. She has a special interest in medical education and student support, including post-exposure prophylaxis.
IIIDr Murray Louw co-ordinates the training of clinical associates and manages healthcare students after accidental exposures to blood-borne pathogens at the University of Pretoria. He is a family physician experienced in providing antiretroviral therapy and in rural medicine.
IVDr Karin Richter is a clinical virologist/consultant pathologist and senior lecturer in the Department of Medical Virology, University of Pretoria and National Health Laboratory Service. Her passion is the prevention of infectious diseases and empowerment through knowledge and education.
ABSTRACT
The risks associated with infection of healthcare workers and students with blood-borne pathogens, specifically HIV, hepatitis B virus and hepatitis C virus, are often neglected. South Africa (SA) currently has no official policies or guidelines in place for the prevention and management of these infections. This article reviews the available data and international guidelines with regard to infected healthcare practitioners and makes minimum recommendations for the SA setting.
The occupational risk of blood-borne infection in healthcare workers and students - collectively termed healthcare professionals (HCPs) - is a significant yet under-researched area in medical practice, especially in the developing world. Although many viral pathogens have been associated with occupational exposure, three are known to pose the most serious risk: HIV, hepatitis B virus (HBV) and hepatitis C virus (HCV). The route of transmission can be percutaneous or mucosal and is related to the work environment and practices of HCPs.1
Importantly, not only are HCPs at risk of acquiring these infections, but once infected they also pose a risk to patients. This has serious policy implications and raises significant ethical challenges. HCPs and patients in developing countries, especially South Africa (SA), are particularly vulnerable to occupational and nosocomial exposure because the prevalence of HIV and HBV is much higher than in the developed world. The work environment may contribute further to this risk, because injection routes are frequently used for administration of medication and improper venesection practices and inadequate facilities for sharps disposal are common.1, 2 Medical and dental students and junior doctors are at high risk owing to their developing skills level, frequent exposure to invasive procedures and long working hours. Two studies in large teaching hospitals in SA reported that 55% and 64% of interns, respectively, reported one or more episodes of occupational exposure. Exposures were more common among first- than second-year interns (62% v. 38%), and only 64% of percutaneous injuries involving HIV-infected blood were reported.3, 4
Many international bodies have developed guidelines for the prevention and management of infection with blood-borne viruses (BBVs) in HCPs. SA, however, does not have management guidelines in place, and large disparities in disease burden, work practices and healthcare resources complicate adoption of international guidelines. This article attempts to frame BBVs in the local context and suggests strategies for prevention, reporting and management responsibilities in a developing-world context.
Prevalence and transmission risk
The exact prevalence of BBV infection in SA HCPs is unknown, but is estimated to mimic that of the general population: HBV 0.2 - 16%, HIV 17.9% and HCV ~2.4%. The risk of occupational infection is highest for HBV (30%), followed by HCV (1 - 2%) and HIV (0.3%). It has been estimated that globally 66 000 HCPs have been infected with HBV through occupational exposure, 1 000 with HIV and 16 000 with HCV.5 Rare cases of patients contracting HIV from infected HCPs have been documented, but the exact risk of provider-to-patient transmission has not been quantified.6Estimated figures are derived from settings of low HCP HIV prevalence (0.4% and 0.7%), and figures may well be higher in settings where high prevalence is coupled with late diagnosis. Transmission of BBVs is associated with exposure-prone invasive procedures (EPPs) (Table 1), inadequate infection control precautions and drug diversion by HCPs who abuse injection drugs, and determined by the circulating viral burden.7
Prevention
Standard universal precautions (Table 2) should always be followed, regardless of the perception of risk of the procedure or the patient. The use of safety-engineered devices such as retractable syringes, needle-free intravenous systems and winged butterfly needles is encouraged. The following disease-specific measures are also advised.
Hepatitis B virus
Proof of immunity (arbitrarily defined as a hepatitis B surface antibody (anti-HBs) level >10 IU/L) or knowledge of infection status should be a mandatory requirement for all HCPs. HCPs are required to be vaccinated against HBV before they start their training, but no compulsory systems are in place to document immunity or to exclude pre-existing chronic HBV infection. HBV vaccination is approximately 92% effective in immunocompetent adults <40 years of age, and only 84% effective in those aged ≥40 years.7 All vaccine recipients with anti-HBs <10 IU/L after the primary vaccine series should be investigated for chronic HBV infection (hepatitis B surface antigen) and non-vaccine exposure (hepatitis B core antibody (anti-Hbc)). If both tests are negative, a second series of single or double vaccine doses can be given.8 If anti-HBs remains <10 IU/L after the second vaccine series, the individual is classified as a non-responder and should receive hepatitis B-specific immunoglobulin after exposure to a known HBV-infected individual.8
As from 2014, a new cohort of potentially Extended Program on Immunization (EPI)-vaccinated healthcare students started their training. Only 16% of persons vaccinated at age <1 year are estimated to have detectable anti-HBs ≥10 mIU/mL 18 years later. However, they generally show good immunological memory, with 60 - 97.4% showing protective anti-HBs levels after a booster dose of HBV vaccine, and are then considered protected.8 , 9
HIV
In 2006, patients with HIV-related diseases occupied more than half of the hospital beds in sub-Saharan Africa, and in SA, even in the era of widely available antiretroviral therapy (ART), at least 44% of medical admissions are of HIV-infected patients.10 Post-exposure prophylaxis (PEP) is advised in all cases of occupational exposure with perceptible risk. The Southern African HIV Clinicians Society 2008 PEP guidelines11 recommend the use of two nucleos(t)ide reverse transcriptase inhibitors together with either a non-nucleoside reverse transcriptase inhibitor (NNRTI), efavirenz, or a protease inhibitor (PI), lopinavir/ritonavir. The newly revised 2013 US Public Health Services PEP guidelines12 advise the use of tenofovir (TDF) and emtricitabine together with the integrase strand transfer inhibitor raltegravir (RAL). This regimen is effective and better tolerated than NNRTI- and PI-based regimens. RAL has the additional advantage that restricted availability limits the likelihood of drug resistance. A combination of zidovudine (AZT)/lamivudine (3TC)/RAL can be used in HCPs with pre-existing renal disease, and an HIV expert should be consulted in cases of pregnancy, breastfeeding, serious medical disease in the HCP, and known or suspected HIV drug resistance in the source patient.
It is vital to ensure that the full 28-day course is completed. This can be achieved through active management of side-effects and anxiety.11 Follow-up is essential and should specifically address condom use, as well as the timing of subsequent HIV and hepatitis tests. Post-PEP HIV testing should be performed by serial enzyme-linked immunosorbent assay (ELISA) testing, and there is currently no consensus on the use of polymerase chain reaction testing in this setting. A case may be made for the use of the HIV viral load (VL) for testing HCPs presenting with possible acute HIV infection after exposure to high-risk patients. These tests do, however, have several limitations such as a window period (albeit shorter than for ELISA) and considerable cost.
Finally, it is essential to exclude active HBV in all HCPs on PEP, because the effect of withdrawing TDF or 3TC after 1 month of treatment in the setting of active HBV is uncertain.
Hepatitis C virus
There is currently no vaccine or effective PEP to protect against HCV after exposure. Effective treatment of HCV is available, however, and it is important to identify and document HCV exposure and monitor for acute infection.
Management of HCPs infected with HBV/HIV/HCV
The optimal management of HCPs infected with BBVs has always been controversial because so few cases have been documented and randomised controlled clinical trials are not feasible. Management is further complicated by the absence of a comprehensive SA policy or guideline. By default, cases are managed on an ad hoc basis and monitoring is sparse or absent. Using international guidelines as a point of reference, with due cognisance of the local context, we suggest specific management strategies in the following sections, as well as in Table 3.
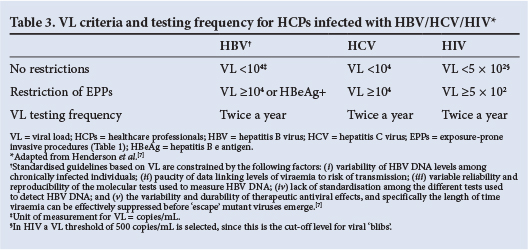
Any management programme should start with acknowledging the importance of HCPs knowing their infection status with respect to all three BBVs, and their obligation to know it, especially when performing EPPs.7 This approach allows for protection of patients from nosocomial transmission, but also enables appropriate and timely access to care for HCPs. HCPs are at greater risk of active tuberculosis (TB), as well as drug-resistant TB, than the general population, a situation exacerbated by the presence of immunodeficiency.13 , 14 In SA, owing to the burden of infectious diseases and the general lack of adequate infection control practices, HCPs are exposed to extraordinary risk in their work environment and every effort should be made to protect them.
Once HCPs are aware that they are infected with one of the BBVs, they should be supported by the medical fraternity, e.g. through expert review panels in their institution or health department or by a designated specialist in the field.7The role of such a panel or expert should be supportive, not punitive, and they should be governed by the professional rules of confidentiality and non-discrimination. Expert advisers can assist infected students and practitioners in minimising the risk of transmission and disease progression by advising on appropriate treatment, monitoring and infection control practices.
International guidelines advise monitoring of infectivity by means of DNA serum levels, i.e. VL, with restriction of EPP above a certain cut-off point (Table 3). Activities not classified as exposure prone are not restricted, provided the HCP does not have a medical condition, such as HIV-related neurocognitive dysfunction, resulting in the inability to perform tasks; there is no prior evidence of transmission of a BBV by the HCP to a patient; and the HCP follows standard infection control guidelines, is able to perform regular duties, and is closely monitored by an expert review panel, occupational safety staff and a physician. Importantly, infection with any of the three BBVs per se is not viewed as sufficient to warrant preclusion of the study or practice of medicine.7 , 15
Reporting and management responsibilities
Neither the Health Professions Council of South Africa nor the South African Medical Association (SAMA) oblige HCPs to know their HIV infection status or disclose this status to an employer. Both encourage voluntary counselling and testing after an exposure incident.16 , 17 Infected practitioners are encouraged to seek counselling from an 'appropriate professional source' familiar with current recommendations, who can advise on the need for restricting professional practice. The SAMA guidelines stress the importance of upholding confidentiality, especially in healthcare institutions, and the right to non-discrimination, as delineated in the SA Constitution (1996) and the Employment Equity Act.17
The ethics of managing infected HCPs are complex and will not be discussed in detail. The debate is strongly influenced by the ethical, professional and fiduciary responsibility HCPs have towards their patients. Suffice it to say that balance should be sought between the HCP's right to confidentiality and non-discrimination and the patient's right to non-maleficence and a safe environment.
While we support voluntary and confidential testing of HCPs, we argue that structures and procedures should be in place to facilitate such testing and that the individual should not be held responsible for the cost of testing. Ideally, institutions should have an expert review panel, or at minimum an occupational health officer, to monitor testing and take responsibility for follow-up of infected HCPs. Restriction of scope of practice should be evidence based and should not be applied if the HCP is adequately treated and is able to practise safely and competently.
An HCP who has been the source of patient exposure should report such an exposure to the occupational health officer and undergo testing for infection with BBVs. The patient should be informed of the exposure and of the outcome of the source's BBV results, and be offered counselling and PEP as appropriate.7 In order to protect confidentiality and in line with international guidelines, patients need not be informed of the name of the source or the exact circumstances of the exposure. Pre-notification of patients regarding their HCP's infection status is also not indicated, provided infection is appropriately managed.
In addition, the following precautions are advised in HIV-infected HCPs: (i) screen for active TB every 6 - 12 months; (ii) isoniazid prophylactic therapy (treat tuberculin skin test (TST)-negative HCPs for 6 months and TST-positive HCPs for 18 months); (iii) pneumococcal vaccine as per current recommendations;18 and (iv) influenza vaccine annually.
HIV-infected healthcare students deserve further mention. Given that students are more likely to experience exposure incidents, they should be offered special assignments while working in high-risk environments such as surgery and TB wards. They should be allowed to withdraw without penalty from any clinical setting they feel poses a high risk of transmission. Students who are not on optimal treatment should be encouraged to seek such treatment, and VL results and ART regimen changes should be reported to the dean or a designated person on an annual basis. The teaching institution should assist students in selecting career paths best suited to their specific situation, and students whose HBV, HCV and/or HIV cannot be effectively cleared or suppressed below the recommended thresholds should be encouraged to select careers that do not involve the highest-risk procedures.
Recommendations
In the absence of SA guidelines, we suggest the following basic principles as minimum requirements:
- All healthcare providers and all healthcare students should know their infection and immune status (as appropriate) for all three major BBVs.
- All HCPs not infected with HBV should be vaccinated and have their immune status confirmed prior to initiation of training. Chronic HBV infection must be excluded in non-responders.
- HCPs infected with HIV, HBV or HCV should seek treatment and obtain expert advice, whether through an institutional expert review panel, occupational health officer or specialist in the field.
- Institutions and healthcare facilities should be familiar with current international guidelines on the management of occupational exposures.
- All institutions should have a dedicated person, such as an occupational health officer, who can monitor and support infected HCPs.
- All occupational injuries need to be documented and the data used to adapt training programmes and introduce innovative ways to prevent such injuries.
- HCPs should have easy and confidential access to testing and treatment for all three BBVs in their place of work.
- PEP should be individualised as more patients are on ART and should be accompanied by adequate medical and psychological support.
REFERENCES
1. Deuffic-Burban S, Delarocque-Astagneau E, Abiteboul D, et al. Blood-borne viruses in health care workers: Prevention and management. J Clin Virol 2011;52(1):4-10. [http://dx.doi.org/10.1016/j.jcv.2011.05.016] [ Links ]
2. Mendelson M, Meintjies G. Increasing the risk of nosocomial transmission of HIV: Pitfalls and practices at a busy secondary level hospital with a high burden of HIV. South Afr J Epidemiol Infect 2009;24(1):8-11. [ Links ]
3. Karani H. Occupational exposure to blood-borne or body fluid pathogens among medical interns at Addington Hospital, Durban. S Afr Fam Pract 2011;53(5):462-466. [http://dx.doi.org/10.1080/20786204.2011.10874135] [ Links ]
4. Karstaedt AS, Pantanowitz L. Occupational exposure of interns to blood in an area of high HIV seroprevalence. S Afr Med J 2001;91(1):57-61. [ Links ]
5. Pruss-Ustun A, Rapiti E, Hutin Y. Estimation of the global burden of disease attributable to contaminated sharps injuries among health-care workers. Am J Ind Med 2005;48(6):482-490. [ Links ]
6. Centers for Disease Control and Prevention. Surveillance of occupationally acquired HIV/AIDS in healthcare personnel, as of December 2010. http://www.cdc.gov/HAI/organisms/hiv/Surveillance-Occupationally-Acquired-HIV-AIDS.html (accessed 12 April 2013). [ Links ]
7. Henderson DK, Dembry L, Fishman NO, et al. SHEA guideline for management of healthcare workers who are infected with hepatitis B virus, hepatitis C virus, and/or human immunodeficiency virus. Infect Control Hosp Epidemiol 2010;31(3):203-232. [http://dx.doi.org/10.1086/650298] [ Links ]
8. Centers for Disease Control and Prevention. CDC guidance for evaluating health-care personnel for hepatitis B virus protection and for administering postexposure management. MMWR Morb Mortal Wkly Rep 2013;62(10):1-19. [ Links ]
9. Chiara F, Bartolucci GB, Mongillo M, et al. Hepatitis B vaccination at three months of age: A successful strategy? Vaccine 2013;31(13):1696-1700. [http://dx.doi.org/10.1016/j.vaccine.2013.01.046] [ Links ]
10. Long L, Sauls C, Sanne I, Rosen S. HIV-related burden on South African hospitals in the era of large-scale access to antiretroviral therapy. Abstract Z - 140, CROI 2012. http://www.retroconference.org/2012/PDFs/659.pdf (accessed 12 April 2013). [ Links ]
11. Andrews S, Mendelson M, Hefer E, et al. Southern African HIV Clinicians Society post-exposure prophylaxis guidelines. Southern African Journal of HIV Medicine 2008;9(1):36-45. [ Links ]
12. Kuhar DT, Henderson DK, Struble KA, et al. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol 2013;34(9):875-892. [http://dx.doi.org10.1086672271] [ Links ]
13. Menzies D, Joshi R, Pai M. Risk of tuberculosis infection and disease associated with work in health care settings. Int J Tuberc Lung Dis 2007;11(6):593-605. [ Links ]
14. O'Donnell MR, Jarand J, Loveday M, et al. High incidence of hospital admissions with multidrug resistant and extensively drug resistant tuberculosis among South African health care workers. Ann Intern Med 2010;153(8):516-522. [http://dx.doi.org10.73260003-4819-153-8-201010190-00008] [ Links ]
15. Centers for Disease Control and Prevention. Updated CDC recommendations for the management of hepatitis B virus-infected health-care providers and students. MMWR Morb Mortal Wkly Rep 2012;61(No. RR-3):1-12. [ Links ]
16. Health Professions Council of South Africa. Guidelines for Good Practice in the Health Care Professions. Ethical Guidelines for Good Practice with Regard to HIV. Booklet 11. Pretoria: HPCSA, May 2008. [ Links ]
17. South African Medical Association. Human Rights and Ethical Guidelines on HIV and AIDS: A Manual for Medical Practitioners. Pretoria: SAMA, 2006. [ Links ]
18. Souter J. An update on pneumococcal vaccination in children and adults. S Afr Pharm J 2014;81(2):15-18. [ Links ]
19. Centers for Disease Control and Prevention. Recommendations for prevention of HIV transmission in health-care settings. MMWR Morb Mortal Wkly Rep 1987;36(25):S3-S18. [ Links ]
 Correspondence:
Correspondence:
K L Richter
karin.richter@up.ac.za
Accepted 1 July 2014.














