Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SAMJ: South African Medical Journal
versión On-line ISSN 2078-5135
versión impresa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.104 no.3 Pretoria mar. 2014
CONTINUING MEDICAL EDUCATION
ARTICLE
The paediatric neuropathic bladder
J Lazarus
MB ChB, MMed Urology, MA, FCUrol (SA). Division of Urology, Red Cross War Memorial Children's Hospital, University of Cape Town, South Africa
ABSTRACT
Paediatric neuropathic bladder dysfunction can cause irreversible renal damage and urinary incontinence. Aetiologically, this condition is usually caused by a congenital neural tube defect. The majority of affected children can be successfully managed with the standard medical treatment of clean intermittent catheterisation and anticholinergic medication (typically oxybutynin). A small subset of patients who receive oral oxybutynin experience severe side-effects or insufficient suppression of detrusor overactivity. This group may require alternative medical or surgical management.
A neurogenic bladder can be defined as a dysfunctinal urinary bladder caused by disease of the central nervous system or peripheral nerves involved in the control of micturition (urination). In pathophysiological terms, a neurogenic bladder is caused by a spinal reflex arc that occurs when the bladder becomes autonomous from higher centres.!1' Paediatric neurogenic bladder dysfunction is most commonly caused by a congenital neural tube defect (NTD), such as myelomeningocele (MMC) or another form of tethered spinal cord. Alternatively, it can be the result of traumatic spinal cord injury, transverse myelitis or anorectal malformation. The standard conservative management of paediatric neurogenic bladder comprises clean intermittent catheterisation and oral anticholinergic medication. This article covers the available literature on the ideal assessment of and contemporary treatment options for this vulnerable group of children.
Physiology of micturition
The normally functioning lower urinary tract brings about low-pressure bladder storage and continence, followed by periodic, complete voluntary voiding. Hence, the micturition reflex is best divided into two discrete phases, i.e. bladder filling/urine storage and bladder emptying/voiding.
Micturition and storage are functions of the parasympathetic and sympathetic nervous systems, respectively. Both are therefore functions of the autonomic nervous system, while the somatic nervous system controls the external urinary sphincter, allowing continence.
The normal micturition reflex involves switching from inhibition of the voiding reflex and activation of storage reflexes to inhibition of the storage reflexes and activation of the voiding reflex - and back again.
Assessment
Many children with a neurogenic bladder have urinary incontinence and potential progressive renal damage because of high bladder storage pressures, secondary vesico-ureteric reflux and recurrent urinary tract infections (UTIs). Children at risk of renal damage should undergo a thorough medical history (including a bladder diary), neurological examination, urinalysis and culture to identify infection. Serial renal ultrasonography allows the renal tract to be monitored and cystography can be used to reveal the configuration of the bladder and detect vesico-ureteric reflux that occurs as a secondary phenomenon in the neurogenic bladder. Nuclear imaging, glomerular filtration rate estimation and, at a later stage, serum creatinine level, can also be used to assess kidney function.
Urodynamic assessment is essential for the diagnosis and prognosis of paediatric neurogenic bladder. Urodynamic studies (UDSs) are functional studies of the lower urinary tract; they evaluate storage and emptying functions of the bladder. UDSs can be simple and non-invasive (bladder diary and flow rate) or invasive (cystometrogram and video-urodynamics). A cystometrogram measures the relationship between bladder filling and pressure. Parameters used to characterise the bladder are capacity, compliance, detrusor activity and sphincter activity (Fig.1).
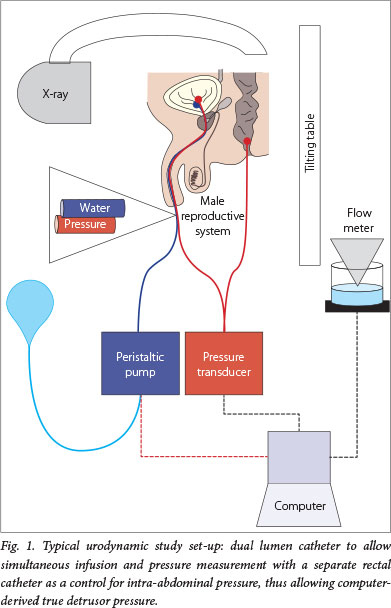
On the other hand, invasive UDSs are indicated to evaluate and characterise the neuropathic bladder. UDSs should be used early in the management and repeated to avoid upper tract deterioration. Paediatric patients with MMC should first undergo UDSs in the neonatal period, but this service is rarely available in South Africa (Fig. 2). However, as it is a test essential to the quality care of these children and adults, UDSs should be fostered nationally.
Risk factors signalling a poor prognosis include:
• reduced bladder capacity for age
• poor compliance
• elevated detrusor leak point pressure (>40 cmH2O)
• features of functional bladder outlet obstruction on electromyography, such as detrusor sphincter dyssynergia.[2]
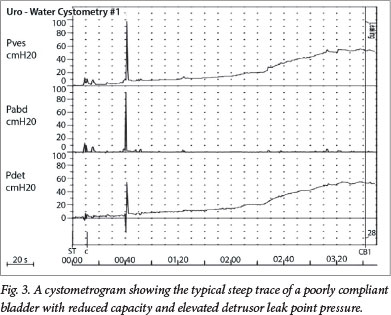
Fig. 3 demonstrates the cystometrogram of a patient with a neuropathic bladder secondary to MMC. It shows, from top to bottom, the vesical, abdominal, and detrusor (computer-calculated) traces. Compared with a normal cystometrogram, there is a steep rise in the pressure v. time trace with bladder filling, representing a typical poorly compliant bladder, where the capacity is reduced and the detrusor leak point pressure is elevated above the danger threshold of 40 cmH2O. Above this value, urinary flow from the kidneys is impaired. Additionally, there are small waves of detrusor instability throughout the study, bladder sensation is reduced and incontinence occurs.
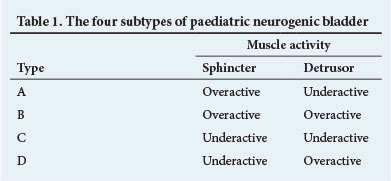
The UDS bladder dysfunction pattern can be dynamic, and warrants close monitoring and life-long follow-up. This diagnostic assessment allows each child to be categorised into one of four subtypes of neuropathic bladder dysfunction based on sphincter and detrusor muscle activity (Table 1).[3] Although these categories may overlap, they assist in patient management. Anticholinergic drugs might at best be expected to treat the neurogenic detrusor overactivity associated with types B and D by improving functional capacity and reducing filling pressures. Types A and C usually require clean intermittent catheterisation alone, with marginal benefit expected from anticholinergics.
Management
The primary goals of paediatric neurogenic bladder management are to normalise vesical pressure and to achieve social continence.
Additionally, 58% of children with untreated MMC exhibit progressive renal deterioration by 3 years of age;[4] therefore the early treatment of these children can also improve renal function.[5]
Pharmacotherapy
During normal bladder function, acetylcholine released from parasympathetic nerves activates muscarinic receptors in this organ, causing detrusor contraction and resultant micturition. In patients with a neuropathic bladder, disordered innervation commonly results in dyssynergia between the detrusor and external sphincters, which adversely affects bladder function.
There are five subtypes of muscarinic receptors expressed in the bladder. Of these, M2 receptors are the most abundant, although M3 receptors are more functionally active.[6] Oral anticholinergics, such as oxybutynin, block these receptors, causing relaxation of the bladder's smooth muscle, and are well established as the firstline pharmacotherapeutic agents for neurogenic bladder in adults and children. The majority of children with neurogenic bladder (70 - 90%) are effectively managed with oral anticholinergics and clean intermittent catheterisation, avoiding the need for surgical intervention, e.g. augmentation cystoplasty or bladder outlet surgery.[7]
Of the anticholinergic agents available, few have regulatory approval for use in children. Oxybutynin chloride is FDA approved. It is widely used, and long-term treatment supports its safety in children, including newborns.[3] The limitations of oral anticholinergic use are the associated side-effects and that a subset of children fail to adequately respond to this therapy. Inadequate responses are characterised by ongoing urinary incontinence, which can reduce quality of life and cause progressive upper-tract dilatation and loss of renal cortical mass. Side-effects of anticholinergic agents include dry mouth, blurred vision, constipation, facial flushing and dizziness. Of significant concern are the suggested cognitive side-effects, particularly as these children represent a neurologically vulnerable cohort.[8]
For children who are not controlled by anticholinergic therapy and clean intermittent catheterisation, other options need to be explored, as discussed below. These patients may have persistent hydronephrosis, new renal scarring, vesico-ureteric reflux, persistent incontinence or UTIs.
While immediate-release oxybutynin is widely used in children with neuropathic bladders, innovative drug delivery systems hold the potential to improve clinical outcomes. Extended-release oxybutynin and tolteridine are also available. Transcutaneous extended-release oxybutynin has been shown in a large trial to be well tolerated, with few undesired anticholinergic side-effects, apart from some skin reactions. Furthermore, new-generation anticholinergic agents, such as darifenacin and solifenacin, are selective for the M3 receptor and thus offer the potential of reduced side-effects. However, few of these newer drugs have regulatory approval for paediatric use.
Brendler et al.[9] were the first to document the intravesical delivery of oxybutynin in adult patients with persistent urge incontinence. The authors claimed that the increased efficacy and reduced side-effect profile observed with intravesical administration were due to lower plasma drug levels. Counter-intuitively, the serum levels of oxybutynin after intravesical administration are as high as when taken orally.[1] However, since first-pass liver metabolism is circumvented with this approach, a reduced side-effect profile is observed. Side-effects are predominantly thought to be mediated by the metabolite N-desethyl oxybutynin.[10]
Botulinum toxin type A injection into the detrusor muscle was first reported in 2000 and has since been widely studied. The neurotoxin binds to the presynaptic nerve endings of cholinergic neurons, resulting in a marked reduction in bladder contractions. The efficacy of botulinum toxin type A injections in children with neurogenic bladders due to spina bifida was described in 2002; 85 - 300 U of botulinum toxin type A was injected into the detrusor muscle, causing an increase in average maximum bladder capacity and detrusor compliance, and a decrease in the mean maximal detrusor pressure. Continence was restored in all 17 children investigated.[1] A further study demonstrated a reduced requirement for oral medication and improved quality of life for children treated with botulinum toxin type A.[11] Urodynamic efficacy lasts for about 6 months, after which repeated injections are necessary.
Clean intermittent catheterisation
Clean intermittent catheterisation is a cornerstone of lifelong management. It is a safe and an effective method of completely emptying the bladder, improving or eliminating urinary incontinence, reducing UTIs and protecting (along with anticholinergics) kidney function.
Clean intermittent catheterisation should be done 3-hourly. The Credé manoeuvre (manual suprapubic pressure to expel urine) is discouraged.
Surgery
Surgical options for failure of conservative treatment typically use a segment of bowel to enlarge the bladder (augmentation cystoplasty), which increases capacity and reduces pressure.
For children with an underactive sphincter (Table 1) who are incontinent with a good-capacity and low-pressure bladder, bladder outlet surgery, e.g. an artificial urinary sphincter, may be contemplated.
Conclusion
Children with neuropathic bladders resulting from MMC require life-long surveillance and treatment, ideally in a multidisciplinary environment. South African medical services need to rise to this challenge and render appropriate care to this vulnerable group of patients.
Some of the information in this article is drawn from a previous publication by the author.[12]
References
1. Reitz A, Schurch B. Intravesical therapy options for neurogenic detrusor overactivity. Spinal Cord 2004;42:267-272. [http://dx.doi.org/10.1038/sj.sc.3101584] [ Links ]
2. Kurzrock EA, Polse S. Renal deterioration in myelodysplastic children: Urodynamic evaluation and clinical correlates. J Urol 1998;159:1657-1661. [http://dx.doi.org/10.1097/00005392-199805000-00084] [ Links ]
3. Verpoorten C, Buyse GM. The neurogenic bladder: Medical treatment. Pediatr Nephrol 2008;23:717-725. [http://dx.doi.org/10.1007/s00467-007-0691-z] [ Links ]
4. Smith ED. Urinary prognosis in spina bifida. J Urol 1972;108:815-817. [ Links ]
5. Dik P, Klijn AJ, van Gool JD, de Jong-de Vos van Steenwijk CC, de Jong TP. Early start to therapy preserves kidney function in spina bifida patients. Eur Urol 2006;49:908-913. [http://dx.doi.org/10.1016/j.eururo.2005.12.056] [ Links ]
6. Chapple CR, Yamanishi T, Chess-Williams R. Muscarinic receptor subtypes and management of the overactive bladder. Urology 2002;60:82-88. [http://dx.doi.org/10.1016/S0090-4295(02)01803-4] [ Links ]
7. Amend B, Hennenlotter J, Schäfer T, Horstmann M, Stenzl A, Sievert KD. Effective treatment of neurogenic detrusor dysfunction by combined high-dosed antimuscarinics without increased side-effects. Eur Urol 2008;53:1021-1028. [http://dx.doi.org/10.1016/j.eururo.2008.01.007] [ Links ]
8. Donnellan CA, Fook L, McDonald P, Playfer JR. Oxybutynin and cognitive dysfunction. Br Med J 1997;315:1363-1364. [http://dx.doi.org/10.1136/bmj.315.7119.1363] [ Links ]
9. Brendler CB, Radebaugh LC, Mohler JL. Topical oxybutynin chloride for relaxation of dysfunctional bladders. J Urol 1989;141:1350-1352. [ Links ]
10. Buyse G, Waldeck K, Verpoorten C, Björk H, Casaer P, Andersson KE. Intravesical oxybutynin for neurogenic bladder dysfunction: Less systemic side effects due to reduced first pass metabolism. J Urol 1998;160:892-896. [http://dx.doi.org/10.1097/00005392-199809010-00084] [ Links ]
11. Ehren I, Volz D, Farrelly E, et al. Efficacy and impact of botulinum toxin A on quality of life in patients with neurogenic detrusor overactivity: A randomised, placebo-controlled, double-blind study. Scand J Urol Nephrol 2007;41:335-340. [http://dx.doi.org/10.1080/00365590601068835] [ Links ]
12. Lazarus J. Intravesical oxybutynin in the pediatric neurogenic bladder. Nat Rev Urol 2009;6(12):671-674. [http://dx.doi.org/10.1038/nrurol.2009.214] [ Links ]
 Corresponding author:
Corresponding author:
J Lazarus
(j.lazarus@uct.ac.za)














