Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.104 n.3 Pretoria Mar. 2014
CONTINUING MEDICAL EDUCATION
ARTICLE
Aetiology and antenatal diagnosis of spina bifida
K FieggenI; C StewartII
IMB ChB, FCPaed (SA), Cert Med Genet (SA). Division of Medical Genetics, University of Cape Town, South Africa
IIMB ChB, MMed, MA, FCOG (SA). Department of Obstetrics and Gynaecology, University of Cape Town and Groote Schuur Hospital, Cape Town, South Africa
ABSTRACT
Spinal neural tube defects (NTDs) result from failure of neural tube closure that normally occurs at 15 - 28 days after conception. Birth prevalence figures for spina bifida vary, but on average are around 0.1%. There are no recent figures for South Africa, but previous studies have shown an incidence of 0.77 - 6.1/1 000 live births, with higher incidences in rural areas. The true incidence of NTDs is thought to be higher, but is difficult to calculate as this includes both spontaneous and therapeutic pregnancy losses.
Terminology and definitions
Spinal neural tube defects (NTDs) result from failure of neural tube closure that normally occurs at 15 - 28 days after conception. Commonly known as spina bifida, the commonest type is a meningomyelocele (or myelomeningocele), which comprises a saclike structure that protudes through the vertebral arches and contains meninges, cerebrospinal fluid (CSF) and spinal cord. Meningoceles are rare and consist of a sac containing meninges and CSF, but no nerve tissue, and therefore have no neurological deficit. Spina bifida occulta, or closed NTD, comprises a spectrum - from a simple midline defect of the vertebrae (that usually goes unnoticed) through to complex abnormalities where the cord is tethered to a lipoma.
Epidemiology and aetiology
Birth prevalence figures for spina bifida vary worldwide, but on average are around 0.1%. There are no recent figures for South Africa, but previous studies have shown an incidence of 0.77 - 6.1/1 000 live births, with higher incidences in rural areas.[1] The true incidence of NTDs is thought to be higher, but is difficult to calculate as this includes both spontaneous and therapeutic pregnancy losses.
Isolated NTDs are typically true multifactorial disorders, reflecting a combination of genetic and environmental influences. The strongest risk factor is a positive family history, which is likely to reflect a number of genetic contributors, each with a small associated risk. However, families also share the environment. The recurrence risk for a woman who has had a single child with an NTD is 2 - 5% and increases if more than one child is affected.[2]
Genetic factors
Although NTDs are usually isolated, they are occasionally associated with chromosomal abnormalities, most often trisomy 18, or Mendelian single gene disorders. These syndromic forms of NTD can usually be recognised by the presence of other structural congenital abnormalities and dysmorphic features. It is important to recognise this to allow for accurate recurrence risk estimation and genetic counselling.[3]
The genetic contributors to NTDs are still poorly understood, but include genes involved in the folic acid pathway, in both fetal and maternal metabolism, and genes coding for many aspects of embryological control such as planar cell polarity and ciliogenesis. There is evidence that epigenetic factors play an important role in the aetiology of NTDs, which may help to explain the way in which environmental factors influence embryological development.[4]
Environmental factors
Folic acid
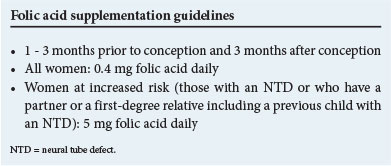
Documentation of folic acid deficiency in a group of mothers with children affected by NTDs led to the discovery that periconceptional supplementation with folic acid could significantly reduce the birth incidence of NTDs by as much as 70%, both as first occurrences and in high-risk women.[5] This was followed by recommendations for folate supplementation and food fortification in a number of countries, including South Africa. Typically, the dose of folic acid obtained through food fortification falls below the recommended dose for women of reproductive age, underscoring the need for additional intake of folate-rich foods or further supplementation, particularly for women at increased risk.
The exact mechanism by which folic acid reduces the risk of NTDs remains unclear and there are women for whom folic acid does not seem to be protective. Folate metabolism is affected by, and interacts with both genetic and environmental factors; this pathway seems to be central in closure of the neural tube.[4]
Teratogens
Anti-epileptic drugs (AEDs) are the most important class of drugs associated with NTDs. Valproic acid has the highest risk -1-2% in exposed pregnancies. This may be in part dose-dependent, and polytherapy is typically associated with a higher risk. Uncontrolled epilepsy also poses a risk to the mother and fetus; therefore change of therapy during an established pregnancy is not recommended. This underscores the role of pre-pregnancy planning and counselling. The goal should be to control epilepsy with the lowest dose of the fewest drugs in women of reproductive age.[6] Folic acid supplementation is recommended in women on AEDs planning or at risk of a pregnancy, also in women taking other folate antagonists, although dosage recommendations vary.
Additional risk factors
Additional risk factors include:
• folate and vitamin B12 deficiency
• poor nutritional status
• maternal diabetes
• maternal obesity
• maternal pyrexia.
Well-documented epidemiological associations include geographical regions, seasonal changes, ethnicity, socio-economic status and parity, which must all, in some way, reflect the complex gene-environment interactions.
Antenatal diagnosis
NTDs are the commonest congenital abnormalities of the central nervous system. With prenatal ultrasound screening, detection of more than 90% of cases is possible.[7]
The current recommendation for prenatal screening for fetal abnormalities is that all women be offered an anatomy scan at 18 - 23 weeks' gestation, which consists of a detailed anatomical survey of the different structures of the fetus. The spine is examined systematically from top to bottom in the sagittal, coronal and transverse planes (Figs 1 - 3). Intactness of the vertebrae is assessed, as well as evidence of a sac-like protrusion. Adequate visualisation is dependent on the quality of the machine, the body mass index of the patient, and the position of the fetus. A fetus lying spine down and very close to the uterine wall may make imaging of the spine difficult. In addition, experience of the operator is an important factor.
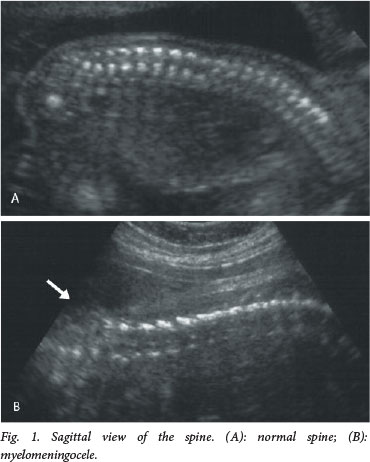

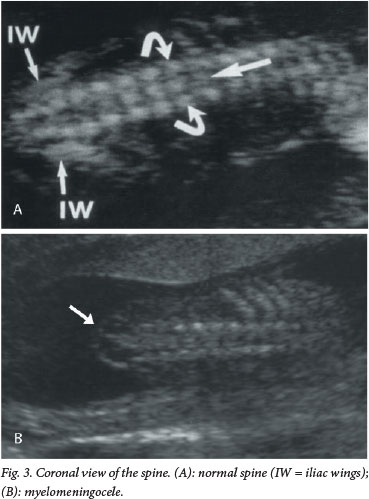
Direct diagnosis of a spinal NTD is often difficult, and more commonly it is suspected when the following typical intracranial features are detected:
• a lemon-shaped head
• the 'banana sign'
• ventriculomegaly.
Instead of the normal concave appearance of the fetal skull, the anterior part of the skull is narrowed, resembling a lemon (Fig. 4). This sign is present in 98% of open spina bifida cases scanned before 24 weeks' gestation.[8] Cerebellar abnormalities are present in 95% of fetuses with open spina bifida scanned before 24 weeks (either the banana sign, where the cerebellum is curved and elongated -not the usual rounded dumb-bell appearance - or absence of the cerebellum). These ultrasound features are due to herniation of the cerebellum into the spinal canal, the so-called Arnold-Chiari II malformation. Although clinically significant hydrocephalus is seldom present at birth, ventriculomegaly is noted antenatally in 86% of cases.[9]

When a diagnosis of spina bifida has been made, one should consider factors that might be prognostic of the functional outcome for the child. The level of the lesion is assessed, as well as its size in terms of the number of segments of the spine involved. The level of the lesion is thought to be the most important prognosticator of motor function.[10] The postnatal functional level correlates with the antenatal ultrasound assessment of the level of the lesion in 60% of cases, with a tendency for the prenatal assessment of the level to be lower than the postnatal level.[11]
Severity of ventriculomegaly is thought to have some prognostic value in terms of intellectual outcome, with measurements of >15 mm being more indicative of some degree of intellectual disability.[12] Neither the absence of talipes nor the presence of leg movement in utero predicts normal motor function.
It is also important to assess whether the lesion is isolated or associated with other abnormalities. This necessitates careful examination of all systems. Chromosomal abnormalities and syndromes will need to be considered in association with other abnormalities.
Because of the potentially severe disability associated with spina bifida, it is important to make the diagnosis at a time in gestation when options are available to the parents. Practitioners not skilled in Level 3 scanning, i.e the expertise to detect and diagnose fetal abnormalities, should refer all women to an appropriately trained practitioner. While this detailed scan is usually done between 18 and 23 weeks, there is some evidence that screening may be done as early as 13 weeks' gestation, where the presence of an intracranial translucency is a reassuring feature that spina bifida is not present.[13]
The role of maternal serum alpha-fetoprotein should not be forgotten. This is raised in open spina bifida. In settings where resources do not permit the use of sufficiently high-resolution ultrasound equipment or where there are no trained personnel, alpha-fetoprotein has an important role in screening for this condition. Occasionally it is difficult to distinguish between an open or a closed spina bifida, and amniotic fluid alpha-fetoprotein and acetylcholinesterase can then be helpful.[14]
Counselling and prevention
Once an antenatal diagnosis of an NTD has been made, prospective parents require both empathy and accurate information about the implications of the diagnosis and options for further management. Such counselling needs to include the following information:
• explanation of the diagnosis
• accurate information on prognosis and outcome
• discussion of the uncertainties that accompany any antenatal diagnosis
• options for further management
• exploration of associated risk factors and information that may carry other implications for the pregnancy
• discussion of recurrence risk and risk reduction for the couple and close family.
Furthermore, the impact of the diagnosis on the family needs careful attention. Exploring support structures and assurance of non-directive support in decision-making are essential.
Further management
In the setting of a condition with severe physical and possible intellectual disability, parents are counselled regarding further management options. These are: (i) to continue the pregnancy; or (ii) to terminate the pregnancy.
Continuation of the pregnancy implies knowledge and acceptance of the challenges for the child and family. Contact with support groups may be helpful. During the course of the pregnancy, ventriculomegaly may increase the size of the head to a level that excludes a vaginal birth. In these cases, the prognosis is usually worse and a caesarean section would be needed to ensure a safe delivery. In the absence of a markedly enlarged head, there is no benefit in elective caesarean section for spina bifida.[15]
Termination of the pregnancy before 24 weeks' gestation involves induction of labour and delivery of a fetus that would be too premature to survive. If the diagnosis is made after 24 weeks, termination of the pregnancy would involve fetocide, with injection of KCl into the fetal heart. This would result in the death of the fetus and induction of labour and delivery can then be planned.
Prevention
Pre-pregnancy counselling and advice to all women should include nutritional information, family planning, teratogen avoidance, optimal control of risk factors, such as maternal obesity and diabetes, and information on periconceptional folic acid supplementation.
Conclusion
Spina bifida remains a common multifactorial birth defect in South Africa. Primary prevention with appropriate folate supplementation and pre-conception counselling to reduce risk factors are essential. Antenatal diagnosis is possible in the majority of cases, allowing women, their families and their caregivers the opportunity to make choices best suited to them and to optimise outcomes.
References
1. Robertson H-L, Steyn NP, Venter PA, Christianson AL. Neural tube defects and folic acid - a South African perspective. S Afr Med J 1997;87(7):928-931. [ Links ]
2. Teckie G, Krause A, Kromberg JG. Neural tube defects in Gauteng, South Africa: Recurrence risks and associated factors. S Afr Med J 2013;103(12):973-977. [http://dx.doi.org/10.7196/SAMJ.7119] [ Links ]
3. Chen CP. Syndromes, disorders and maternal risk factors associated with neural tube defects (I). Taiwan J Obstet Gynecol 2008;47(1):1-9. [http://dx.doi.org/10.1016/S1028-4559(08)60048-0] [ Links ]
4. Copp AJ, Stanier P, Greene ND. Neural tube defects: Recent advances, unsolved questions, and controversies. Lancet Neurol 2013;12(8):799-810. [http://dx.doi.org/10.1016/S1474-4422(13)70110-8] [ Links ]
5. MRC Vitamin Study Research Group. Prevention of neural tube defects: Results of the Medical Research Council Vitamin Study. Lancet 1991;338(8760):131-137. [http://dx.doi.org/10.1016/0140-6736(91)90133-A] [ Links ]
6. Krishnamurthy KB. Managing epilepsy during pregnancy: Assessing risk and optimizing care. Curr Treat Options Neurol 2012;14(4):348-355. [http://dx.doi.org/10.1007/s11940-012-0184-7] [ Links ]
7. Boyd PA, Devigan C, Khoshnood B, et al. Screening policies in Europe for structural malformations and chromosome anomalies, and their impact on detection and termination rates for neural tube defects and Down's syndrome. Br J Obstet Gynaecol 2008;115(6):689-696. [http://dx.doi.org/10.1111/j.1471-0528.2008.01700.x] [ Links ]
8. Van den Hof MC, Nicolaides KH, Campbell J, Campbell S. Evaluation of the lemon and banana signs in one hundred thirty fetuses with open spina bifida. Am J Obstet Gynecol. 1990;162(2):322-327. [http://dx.doi.org/10.1016/0002-9378(90)90378-K] [ Links ]
9. Nicolaides KH, Campbell S, Gabbe SG, Guidetti R. Ultrasound screening for spina bifida: Cranial and cerebellar signs. Lancet 1986;2(8498):72-74. [http://dx.doi.org/10.1016/S0140-6736(86)91610-7] [ Links ]
10. Biggio JR, Owen J, Wenstrom KD, Oakes J. Can prenatal ultrasound findings predict ambulatory status in fetuses with open spina bifida? Am J Obstet Gynecol 2001;185(5):1016-1020. [http://dx.doi.org/10.1067/mob.2001.117676] [ Links ]
11. Appasamy M, Roberts D, Piling D, Buxton N. Antenatal ultrasound and magnetic resonance imaging in localising the level of the lesion in spina bifida and correlation with postnatal outcome. Ultrasound Obstet Gynecol 2005;27(5):530-536. [ Links ]
12. Peralta CB, Bunduki V, Plese JP, et al. Association between prenatal sonographic findings and postnatal outcome in 30 cases of spina bifida aperta. Prenat Diagn 2003;23(4):311-314. [http://dx.doi.org/10.1002/pd.584] [ Links ]
13. Chaoui R, Benoit B, Mitkowska-Wozniak H, et al. Assessment of intracranial translucency (IT) in the detection of spina bifida at the 11-13 week scan. Ultrasound Obstet Gynecol 2009;34:249-252. [http://dx.doi.org/10.1002/uog.7329] [ Links ]
14. Wang ZP, Li H, Hao LZ, Zhao ZT. The effectiveness of prenatal serum biomarker screening for neural tube defects in second trimester pregnant women: A meta-analysis. Prenat Diagn 2009;29(10):960-965. [http://dx.doi.org/10.1002/pd.2325] [ Links ]
15. Bensen JT, Dillard RG, Burton BK. Open spina bifida: Does caesarean section improve prognosis? Obstet Gynecol 1988;1(4):532-534. [ Links ]
 Corresponding author:
Corresponding author:
K Fieggen
(karen.fieggen@uct.ac.za)














