Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SAMJ: South African Medical Journal
versión On-line ISSN 2078-5135
versión impresa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.104 no.3 Pretoria mar. 2014
RESEARCH
High pleural fluid adenosine deaminase levels: A valuable tool for rapid diagnosis of pleural TB in a middle-income country with a high TB/HIV burden
C P OnyenekwuI; A E ZemlinII; R T ErasmusIII
IMB BS. Division of Chemical Pathology, Faculty of Health Sciences, National Health Laboratory Service and Stellenbosch University, Cape Town, South Africa
IIMB ChB, MMed, FCPath (SA) (Chem). Division of Chemical Pathology, Faculty of Health Sciences, National Health Laboratory Service and Stellenbosch University, Cape Town, South Africa
IIIMB BS, FMCPath (Nig), FWACP (WA), DABCC, DHSM (SA), FCPath (SA) (Chem). Division of Chemical Pathology, Faculty of Health Sciences, National Health Laboratory Service and Stellenbosch University, Cape Town, South Africa
ABSTRACT
BACKGROUND: South Africa has the highest burden of tuberculosis (TB) in the World Health Organization (WHO) African region. Using traditional TB diagnostic tools, the diagnosis of pleural TB (PTB) is highly unrewarding. Elevated levels of pleural fluid adenosine deaminase (FADA) have been shown to be useful in the diagnosis of PTB; however, similar levels may be found in some other medical conditions leading to misdiagnosis. Following queries from clinicians concerning the likely high false-positive (FP) rate of FADA from our laboratory, we performed a retrospective audit of all high FADA results generated over a 12-month period.
OBJECTIVES: To determine the positive predictive value (PPV) of FADA, the frequent causes of FPs in our laboratory and the demographic characteristics of tuberculous pleural effusions (TPEs) and non-tuberculous pleural effusions (NTPEs).
METHODS: High FADA results generated in the past year were extracted with corresponding TB culture results, fluid cell count, cytology/ histology results, radiology reports and HIV results. Hospital records were reviewed for the final diagnosis in each case. Diagnosis of PTB was based on the WHO case definition of TB.
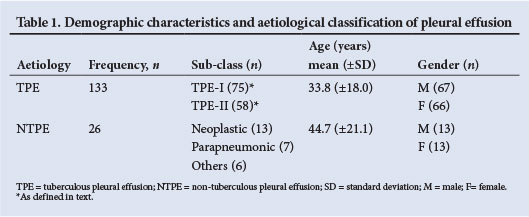
RESULTS: A total of 159 results were reviewed: 133 (83.6%) were TPE, hence FADA had a PPV of 83.6%. Neoplasm was the most common cause of an FP in 13/26 (50%) NTPEs. While TPE was more common than NTPE in younger people, both groups had an equal gender distribution.
CONCLUSION: FADA had a high PPV for PTB in our laboratory. We recommend its continued use as a rapid and reliable diagnostic tool for PTB.
The burden of tuberculosis (TB) in South Africa (SA) is the third highest in the world after China and India and contributes one quarter of the burden of TB in the World Health Organization (WHO) African region.[1] The incidence of TB in the Western Cape is higher than the national incidence rate of 823/100 000 population, at 935/100 000 population[2] Extrapulmonary TB constitutes 14.5% of TB cases in SA.[1] HIV/TB co-infection has been associated with pleural TB (PTB), a form of extrapulmonary TB.[3] Delays in the diagnosis of TB and the initiation of therapy, in addition to the high HIV/TB co-infection rates, are some of the obstacles to the achievement of the sixth millennium development goal in the WHO African region.[1]
The diagnosis of PTB using traditional methods is challenging due to the low sensitivity of routine TB diagnostic tools,[4]viz. sputum smear microscopy (developed over 100 years ago and the most common method for TB diagnosis worldwide), culture for Mycobacterium tuberculosis (the current reference standard for TB diagnosis), and polymerase chain reaction-based techniques and rapid molecular tests (developed more recently for TB diagnosis and diagnosis of drug-resistant TB).[1]
About 35 years ago, Piras et al.[5] first observed that adenosine deaminase (ADA), a zinc-containing metalloenzyme expressed in high levels by lymphocytes and monocytes, was elevated in PTB. Since then, several authors have confirmed this finding, supporting the use of high pleural fluid ADA (FADA) as a simple, rapid, sensitive, cost-effective and robust diagnostic tool for PTB.[6-10] This led to its widespread implementation and routine utilisation in countries such as SA with a high prevalence of TB. However, high FADA levels can also occur in other disease conditions and particularly in empyemas, lymphomas, carcinomas and parapneumonic effusions due to proliferation of large numbers of neutrophils and lymphocytes in these conditions, resulting in false-positive results and misdiagnosis.[11] The usefulness of FADA in the diagnosis of PTB depends on the prevalence of TB. In populations with a high prevalence, the sensitivity and specificity may be as high as 95% and 90%, respectively, but in low prevalence areas, the specificity can be considerably lower.[12]
An essential component of clinical practice is the use of clinical laboratory test results in diagnostic decision-making; hence, there is a need for every diagnostic tool to be reliable, relevant and cost-effective, particularly in a middle-income country where the disease burden is high.
In response to queries from clinicians concerning the possible high false-positive (FP) rate of the FADA test in our laboratory, with many negative TB culture results, we performed a retrospective audit of all the high FADA results generated over a 1-year period.
Objectives
To determine (i) the positive predictive value (PPV) of high FADA test results for PTB; (ii) the most common causes of FPs and the demographic characteristics of tuberculous pleural effusions (TPEs) and non-tuberculous pleural effusions (NTPEs); (iii) the frequency of TPEs in HIV-positive and HIV-negative patients with pleural effusions; and (iv) the FADA levels in TPEs and NTPEs and in HIVpositive and -negative patients.
Methods
Study setting and design
High FADA results generated at the Chemical Pathology Laboratory, Tygerberg Hospital (TH), over a 1-year period from 1 January to 31 December 2012 were reviewed. The hospital serves ~5.8 million people residing within the Western Cape, also serving as a referral centre for many hospitals and primary healthcare clinics in the region. The laboratory at TH is an accredited facility, which serves the hospital and satellite hospitals within the Western Cape; it receives up to 1 000 ADA requests annually, including non-pleural fluid specimens such as cerebrospinal, ascitic, pericardial and synovial fluids.
Ethical approval for the audit was granted by the Health Research and Ethics Committee of Stellenbosch University, Tygerberg, SA. The audit was performed according to the Declaration of Helsinki. As this was a retrospective study and patient consent had been obtained prior to thoracocentesis, the need for individual patient consent for this study was waived by the ethics committee. Patient confidentiality was maintained throughout.
Study criteria included all high (>30 U/l) FADA results generated within the study period, from patients managed within TH, with corresponding TB culture requests. We excluded non-pleural fluid ADA results, empyemas, high FADA results without TB culture results, requests from satellite hospitals and requests with lost patient records.
Specimens for ADA assays were collected in plain tubes and stored at 2 - 8oC before analysis. Laboratory analysis was performed on a weekly basis. Turbid or bloody specimens were centrifuged and haemolysed and grossly purulent samples were rejected.
FADA activity was determined manually by spectrophotometry using the Giusti and Galanti method.[13] It is based on the enzymatic deamination of adenosine to inosine, with the release of ammonia which is determined by Berthelot's reaction. One unit of ADA is defined as the amount of enzyme required to release 1 mmol/min of ammonia from adenosine at standard assay conditions. Each run was performed with a low- and a normallevel quality control material.
FADA results meeting the study criteria were retrieved. Fluid cell count, HIV status, radiological, cytological and histological investigation reports were also extracted, where available. Patient hospital records were reviewed to obtain information on the final diagnosis. A diagnosis of TB was based on the WHO guidelines for case definitions of extrapulmonary TB.[14] The final diagnosis was classified into two broad groups of TPEs and NTPEs, with sub-classifications as follows:
TPEs:
TPE-I: Identification of M. tuberculosis complex in fluid or biopsy specimen by culture; histological evidence of granulomas and positive stain for acid-fast bacilli (AFB) in pleural biopsy; and/ or positive sputum or gastric wash-out culture with clinical and radiological evidence of TB without any other cause for pleural effusion.
TPE-II: The decision by a clinician to treat a patient with a full course of TB chemotherapy, based on strong clinical and radiological evidence, in the absence of other causes of pleural effusion.
NTPEs:
Neoplastic effusions: histological evidence of a malignant effusion or a tumour, in the absence of other causes of pleural effusion.
Parapneumonic effusions: identification of an organism in the pleural fluid or clinical and radiological evidence of pneumonia, with response to antibiotic therapy.
Other: effusions due to conditions such as congestive cardiac failure, chronic liver disease, systemic lupus erythematosus and nephrotic syndrome.
Definition of true-positive (TP) and FP
TP: FADA >30 U/l with the effusion fulfilling the criteria for TPE-I or TPE-II classification as defined above
FP: FADA >30 U/l with the effusion fulfilling the criteria for NTPE classification as defined above.
Calculation of PPV
The PPV of FADA was calculated as: PPV = TP/[TP + FP].
Statistical analysis
Data were analysed using Statistica (version 11.0). Continuous data are expressed as means (± standard deviations (SDs)). One-way analysis of variance (ANOVA) was employed and p<0.05 was considered significant.
Results
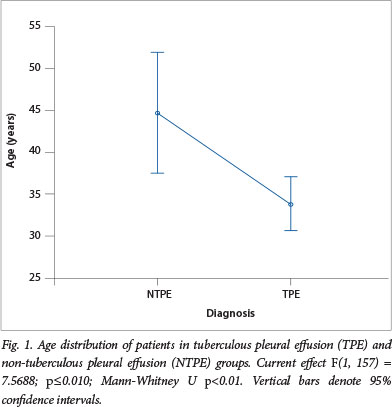
A total of 614 FADA results were requested from within the hospital during the study period; 263 of these were high (>30 U/l). After exclusion of 25 empyematous specimens, 61 requests without corresponding TB microscopy and culture results, 12 duplicate results and 6 lost hospital records, a total of 159 results were then analysed. There were 133 (83.6%) TPE and 26 (16.4%) NTPE, the latter giving FADA a PPV of 83.6% for PTB. Males and females were equally distributed in both groups. A significantly younger age distribution was observed in the TPE group (Fig. 1). Table 1 shows a summary of the mean age and frequency of the 159 results according to aetiological classification and gender.
Employing the lymphocyte/neutrophil ratio, there was a slight but non-significant increase of the PPV of FADA to 89%. The mean (±SD) FADA level in the TPE group was 76.4 (±29.1) U/l and slightly higher than that of the NTPE group 74.1 (±54.9) U/l. This difference was, however, not significant (Fig. 2).

TB culture was positive in 75 (56.4%) of the TPE group (TPE-I), in the remaining 58 (43.6%) TB culture-negative samples, classification as TPE was based on the fulfilment of criteria for TPE-II. Using microscopy techniques for AFB without culture, only 13 (9.8%) cases of TPE were positive.
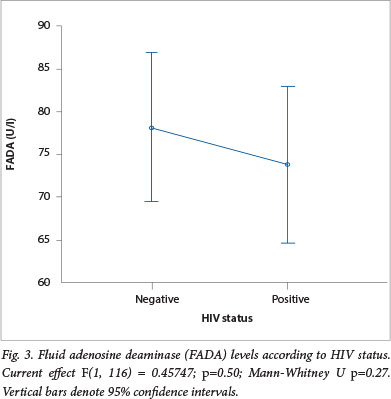
The HIV status was available for 118 (74.2%) patients, 56 (47.5%) of these were HIV-positive. Analysis of HIV/TB co-infection showed that a significantly higher number of 52 (93%) HIVpositive patients were TB-positive compared with 52 (84%) HIV-negative patients who were TB-positive (p<0.05). One-way ANOVA for FADA levels and HIV status showed a mean (±SD) FADA of 74.4 (±36.3) U/l in HIV-positive patients and this was lower than the mean (±SD) FADA of 78.7 (±32.7) U/l observed in the patients who were HIVnegative. This difference was, however, not statistically significant (Fig. 3).
Discussion
The diagnosis of PTB is challenging due to the poor performance of the current gold standard for TB diagnosis - TB culture. Elevated FADA levels have been used as a fast and convenient adjunct diagnostic tool for PTB, particularly in areas with high TB burden. However, other conditions may also cause an increase in FADA levels with a negative TB culture. An effusion may be reactive, in which case the TB culture would be negative due to the absence of bacilli. The effusion may be paucibacillary in nature and the decontamination step performed prior to culture may decrease the number of bacilli, further decreasing the sensitivity of TB culture, resulting in a false-negative. Quality control procedures are put in place for TB culture, using an M. tuberculosis H37RV strain as a positive control and phosphate buffer as a negative control with every batch of specimens at our laboratory. In response to queries from clinicians concerning many TB culture-negative results with high FADA results, we reviewed all high FADA results generated over the study period. Our main objectives were to assess the PPV of FADA for PTB and to determine the conditions that cause FPs and the demographic characteristics of TPE and NTPE with high FADA levels.
We observed a high PPV of 83.6%, which did not change significantly when the lymphocyte/neutrophil ratio was employed alongside, for the diagnosis of PTB. Of the NTPE, 13 (50%) were due to malignancies and nearly half of these were due to bronchial cancer, the other malignancies were single cases of ductal carcinoma of the breast, B cell lymphoma, parotid cancer, carcinoid tumour, basal cell carcinoma and infiltrating thymic tumour. A very poor sensitivity of 9.8% was observed for microscopy for AFB, while the reference standard for TB diagnosis, TB culture, had a sensitivity of 56.4%. TPEs occurred in a relatively younger age group than the NTPEs, but there was an equal gender distribution in the two groups. More HIVpositive patients had TPEs than HIV-negative patients, although their FADA levels were not significantly different.
Our finding of a PPV of 83.6% at a cut-off of 30 U/l was in agreement with that of Burgess et al.,[8] who also reported a PPV of 84%, a negative predictive value (NPV) of 89%, sensitivity of 91% and specificity of 81%, though at a higher cut-off of 50 U/l. Zemlin et al.[10] observed similar diagnostic performance with FADA having a PPV of 85.5%, an NPV of 95.2%, sensitivity of 93.7% and specificity of 88.7% at an ADA cut-off of 52.4 U/l. A much higher PPV of 95% was reported by Riantawan et al. at a cut-off of 60U/l. [9] The nonsignificant increase in the PPV when a lymphocyte/neutrophil ratio of >0.75 was combined with ADA, differs from observations by Burgess et al.[8] who recommended the combined use of ADA with differential fluid cell count, after an observed increase in specificity and PPV, for a more efficient diagnosis of PTB. This differing result may be due to the exclusion of empyematous effusions from our study. Empyemas are a known cause of elevated ADA and usually have a predominance of neutrophils. Nearly 10% of the high ADA results generated in our laboratory during the study period were due to empyemas, which had missed being rejected.
The poor sensitivity observed for the traditional diagnostic methods for PTB in the present study is a well-reported finding that makes diagnosis of PTB a challenge. The implication is that utilisation of either microscopy for AFB alone or TB culture of fluid would lead to many TB-positive patients being missed.
Equal gender distribution seen in the TPE and NTPE groups differs from that of previous studies where there were generally slightly more males than females.[10,15] This may be due to changing patterns in access to healthcare, gender issues and changing epidemiological profiles, such as the rise of HIV infection particularly among women, which may contribute to the development of more TB cases in females.[16] We observed that more patients who were HIVpositive had TPEs than patients who were HIV-negative. This is in agreement with the current epidemiological profile for TB/HIV co-infection rates.[1]
The older age distribution in the NTPE group might have been due to the aetiological conditions in this group, which are more common in older individuals. They consisted mainly of malignancies, parapneumonias and congestive cardiac failure. Most of the malignancies were due to bronchial cancer, which is in agreement with Zemlin et al.[10] Valdes et al.[17] found that parapneumonic effusions were the most frequent reason for a high FADA, followed by carcinomas (with pleural metastatic lesions) and lymphomas.
Study limitations
We reviewed only high FADA results and hence were not able to assess other measures of diagnostic accuracy apart from the PPV. However, previous studies have been carried out in our laboratory to assess the diagnostic accuracy of FADA for PTB,[8,10] which confirmed a high sensitivity and specificity. As the study was retrospective, high ADA results without corresponding microbiology results were excluded. In addition, the HIV status of the patients was not available in all cases. We excluded results of patients from satellite hospitals and clinics owing to difficulty in accessing the patient records from these facilities; however, this is unlikely to affect our findings as these hospitals, located within the Western Cape region, share a similar TB prevalence rate to that of TH.
Despite the limitations, we feel that this study is robust in that it reviews results over 1 year. We utilised the WHO-recognised reference standard for TB diagnosis as the gold standard in assessing for diagnosis of TB throughout.
There is room for a similar but more extensive audit in future, involving FADA results from our hospital, other regional referral hospitals in the country and the satellite healthcare facilities utilising our laboratory services.
Conclusion
This audit has confirmed that high FADA results generated at our laboratory have a good precision rate for PTB. The few FPs that occurred are mostly due to malignancies, which occur in older people and can be diagnosed by cytology/histology and/or radiology, after confirming that the TB culture is negative. We reaffirm the fact that a high FADA is an indispensable TB diagnostic tool and recommend its continued use as a rapid means of diagnosing PTB in a middle-income country with a high TB/HIV burden.
Acknowledgements. The authors are grateful to Mr W Kleinhans for his support with the retrieval of laboratory records and Prof. M Kidd for his assistance with the statistical analysis.
References
1. World Health Organization. Global Tuberculosis Report 2013. Geneva: WHO, 2012. http://www.who.int/tb/publications/global_report/en/ (accessed 7 April 2013). [ Links ]
2. Western Cape Government. World TB Day, March 2012. 23 March 2012. http://www.westerncape.gov.2/news/world-tb-day-24-march-2012 (accessed 7 April 2013). [ Links ]
3. Frye MD, Pozsik CJ, Sahn SA. Tuberculous pleurisy is more common in AIDS than in non-AIDS patients with tuberculosis. Chest 1997;112(2):393-397. [http://dx.doi.org/10.1378/chest.112.2.393] [ Links ]
4. Kataria YP, Khurshid I. Adenosine deaminase in the diagnosis of tuberculous pleural effusion. Chest 2001;120(2):334-335. [http://dx.doi.org/10.1378/chest.120.2.334] [ Links ]
5. Piras MA, Gakis G, Budroni M, Andreoni G. Adenosine deaminase activity in pleural effusions: An aid to differential diagnosis. BMJ 1978;2(6154):1751-1752. [http://di.doi.org/10.1136/bmj.2.6154.1751-a] [ Links ]
6. Martiz FJ, Malan C, Le Roux I. Adenosine deaminase estimation in the differentiation of pleural effusions. S Afr Med J 1982;62(16):556-558. [ Links ]
7. Blake J, Berman P. The use of adenosine deaminase assays in the diagnosis of tuberculosis. S Afr Med J 1982;62(1):556-558. [ Links ]
8. Burgess LJ, Martiz FJ, Le Roux I, Taljaard F. Combined use of pleural adenosine deaminase with lymphocyte neutrophil ratio. Increased specificity for the diagnosis of tuberculous pleuritis. Chest 1996;109(2):414-419. [http://dx.doi.org/10.1378/chest.109.2.414] [ Links ]
9. Riantawan P, Chaowalit P, Wongsangiem M, Rojanaraweewong P. Diagnostic value of pleural fluid adenosine deaminase in tuberculous pleuritis with reference to HIV coinfection and a Bayesian analysis. Chest 1999;116(1):97-103. [http://dx.doi.org/10.1378/chest.116.1.97] [ Links ]
10. Zemlin AE, Burgess LJ, Carstens ME. The diagnostic utility of adenosine deaminase isoenzymes in tuberculous pleural effusions. Int J Tuberc Lung Dis 2009;13(2):214-220. [ Links ]
11. Van Kiempema AR, Slaats EH, Wagenaar JP. Adenosine deaminase, not diagnostic for tuberculous pleurisy. Eur J Respir Dis 1987;71(1):15-18. [ Links ]
12. Khatami K. Pleural Tuberculosis. Shiraz E Medical Journal 2002;3(3):78-86. [ Links ]
13. Giusti G. Adenosine deaminase. In: Bergmayer H-U, ed. Methods of Enzymatic Analysis. 2nd ed. New York: Academic Press, 1974:1092-1099. [ Links ]
14. World Health Organization. Treatment of Tuberculosis: Guidelines. 4th ed. Geneva: WHO, 2010:2324. http://www.who.int/tb/publications/2010/9789241547833/en/ (accessed 11 April 2013). [ Links ]
15. Valdes L, Alvarez D, San José E, et al. Value of adenosine deaminase in the diagnosis of tuberculous pleural effusions in young patients in a region of high prevalence of tuberculosis. Thorax 1995;50:600-603. [http://dx.doi.org/10.1136/thx.50.6.600] [ Links ]
16. Weyer K, R Matji. Epidemiology and Control of Tuberculosis. In: Weyer K, ed. Management of Tuberculosis. Pretoria: Foundation for Professional Development, South African Medical Association, 2004. [ Links ]
17. Valdés L, San José E, Alvarez D, Valle JM. Adenosine deaminase (ADA) isoenzyme analysis in pleural effusions: Diagnostic role, and relevance to the origin of increased ADA in tuberculous pleurisy. Eur Respir J 1996;9(4):747-751. [http://dx.doi.org/10.1183/09031936.96.09040747] [ Links ]
 Corresponding author:
Corresponding author:
A E Zemlin
(azemlin@sun.ac.za)
Accepted 16 January 2014.














