Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SAMJ: South African Medical Journal
versión On-line ISSN 2078-5135
versión impresa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.104 no.3 Pretoria mar. 2014
FORUM
DRUG ALERT
Recommendations pertaining to the use of viral vaccines: Influenza 2014
S Walaza
S Walaza is a medical epidemiologist at the Centre for Respiratory Diseases and Meningitis, National Institute for Communicable Diseases (NICD), Johannesburg, South Africa and has compiled this article on behalf of the Centre for Respiratory Diseases and Meningitis, NICD, Johannesburg, South Africa and the National Department of Health, Pretoria, South Africa
ABSTRACT
Here we provide recommendations for the use of viral vaccines in anticipation of the 2014 southern hemisphere influenza season. For a review of the 2013 influenza season, please refer to the National Institute for Communicable Diseases, National Health Laboratory Service website (http://www.nicd.ac.za).
Recommended vaccine formulation
The following strains have been recommended by the World Health Organization (WHO)[1] for the 2014 southern hemisphere influenza season:
• A/California/7/2009 (H1N1)pdm09-like virus (A/Christchurch/ 16/2010 is an A/California/7/2009-like virus)
• A/Texas/50/2012 (H3N2)-like virus (A/Texas/50/2012 is an A(H3N2) virus that, following adaptation to growth in eggs, has maintained antigenic properties similar to the majority of recently circulating cell-propagated A(H3N2) viruses including A/ Victoria/361/2011)
• B/Massachusetts/2/2012-like virus.
Vaccines should contain 15 ug of each haemagglutinin antigen in each 0.5 ml dose.
Indications
• Persons (adults or children) who are at high risk for influenza and its complications because of underlying medical conditions and who are receiving regular medical care for conditions such as chronic pulmonary and cardiac diseases, chronic renal diseases, diabetes mellitus and similar metabolic disorders; individuals who are immunosuppressed (including HIV-infected persons with CD4+ counts >100 cells/µl); individuals who are morbidly obese (body mass index >40 kg/m2)
• Pregnant women - irrespective of stage of pregnancy
• Residents of old-age homes, chronic care and rehabilitation institutions
• Children on long-term aspirin therapy
• Medical and nursing staff responsible for the care of high-risk cases
• Adults and children who are family contacts of high-risk cases
• All persons >65 years of age
• Any persons wishing to protect themselves from the risk of contracting influenza, especially in industrial settings where large-scale absenteeism could cause significant economic losses.
Dosage
• Adults: Whole or split-product or subunit vaccine: one dose intramuscularly (IM)
• Children (<12 years of age): Split-product or subunit vaccine: one dose IM
• Children (<9 years of age) who have never been vaccinated: two doses, 1 month apart
• Children (<3 years of age): Half the adult dose on two occasions separated 1 month apart
• The influenza vaccine is not recommended for infants <6 months of age.
Contraindications
• Persons with a history of severe (anaphylactic) hypersensitivity to eggs or other components of the vaccine
• Persons with acute febrile illnesses should preferably be immunised after symptoms have disappeared.
Timing
Vaccines should be given sufficiently early to provide protection for the winter. A protective antibody response takes about two weeks to develop.
Antiviral chemotherapy
At present, influenza A (H1N1) pdm09 and H3N2, and influenza B viruses remain sensitive to oseltamivir (and zanamivir). The dosages for treatment and post-contact prophylaxis (where indicated) are provided in Table 1.
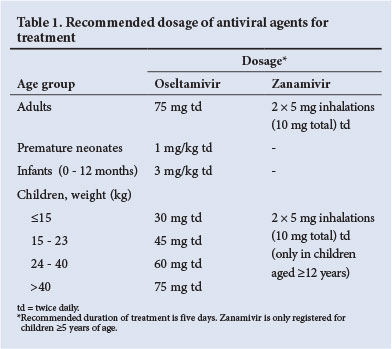
Antiviral chemoprophylaxis
Antiviral chemoprophylaxis for the contacts of persons infected with influenza is currently not recommended. Recent WHO recommendations advise presumptive treatment using the treatment regimen described above for higher-risk individuals (patients with severe immunosuppression or transplant patients) exposed to influenza instead of the previously recommended long-term lower-dose chemoprophylaxis regimen. These higher-risk individuals need to be carefully monitored during the influenza season for early signs and should then be treated immediately on suspicion of infection.
For a more detailed description of antiviral management and chemoprophylaxis of influenza, please refer to the National Institute for Communicable Diseases Healthcare Workers Handbook on Influenza.[2]
For the full report on recommended influenza vaccines, refer to the WHO recommendations.[1]
1. World Health Organization. Recommended Composition of Influenza Virus Vaccines for use in the 2014 Southern Hemisphere Influenza Season. 26 September 2013. http://www.who.int/influenza/vaccines/virus/recommendations/201309_ recommendation.pdf?ua=1 (accessed 30 January 2014). [ Links ]
2. National Institute for Communicable Diseases. Healthcare Workers Handbook on Influenza. Johannesburg: NICD, 1013. http://www.nicd.ac.za/assets/files/Healthcare%10Workers%10 Handbook%20on%20Influenza%20in%20SA%20-%20Final.pdf (accessed 30 January 2014). [ Links ]
 Corresponding author:
Corresponding author:
S Walaza
(sibongilew@nicd.ac.za)
Accepted 30 January 2014.














