Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SAMJ: South African Medical Journal
versión On-line ISSN 2078-5135
versión impresa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.104 no.1 Pretoria ene. 2014
RESEARCH
Critical value reporting: A survey of 36 clinical laboratories in South Africa
E SchapkaitzI; Z MafikaII
IMB BCh, FCPath (Haem), MMed (Haem). Department of Molecular Medicine and Haematology, National Health Laboratory Service and University of the Witwatersrand, Johannesburg, South Africa
IIMB ChB. Department of Molecular Medicine and Haematology, National Health Laboratory Service and University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
OBJECTIVE: Critical value policies are used by clinical laboratories to decide when to notify caregivers of life-threatening results. Despite their widespread use, critical value policies have not been published locally. A survey was designed to determine critical value policies for haematology tests in South Africa.
METHODS: A survey was carried out on 136 identified laboratories across South Africa in January 2013. Of these, 36 responded. Data collected included critical value policies, critical values for haematology parameters, and critical value reporting.
RESULTS: Of the 36 laboratories surveyed, 11.1% (n=4) were private, 33.3% (n=12) were affiliated to academic institutions and 55.6% (n=20) were peripheral or regional National Health Laboratory Service laboratories. All the laboratories confirmed that they had a critical value policy, and 83.3% of such policies were derived from local clinical opinion. Mean low and high critical limits for the most frequently listed tests were as follows: haemoglobin <6 and >20 g/dl, platelet count <41 and >1 000 x109/l, white cell count <2 and >46x109/l, activated partial thromboplastin time >101 seconds, and international normalised ratio >6. In almost all cases critical value reporting was performed by the technologist on duty (97.2%). The majority of laboratories required that the person notified of the critical value be the doctor who ordered the test or the caregiver directly involved in the patient's care (83.3%); 73.3% of laboratories indicated that they followed an algorithm if the doctor/caregiver could not be reached.
CONCLUSION: Each laboratory is responsible for establishing clinically relevant critical limits. Clinicians should be involved in developing the laboratory's critical value policy. The findings of this survey may be of value to local laboratories that are in the process of establishing or reviewing critical value policies.
Critical value policies used by clinical laboratories ensure that caregivers are notified of patients' life-threatening results. Lundberg[1] was the first author to define critical values as results that may lead to adverse outcomes for patients if clinicians were not notified urgently of the critical result. He developed a system whereby laboratories identified critical results and contacted the clinician caring for the patient.[1] Critical value reporting has subsequently become an accreditation requirement, with most laboratories having implemented critical value policies as a quality assurance practice.
Although critical value reporting is widely accepted, it poses certain challenges. Each laboratory is responsible for establishing critical values policies in conjunction with local clinical opinion, review of laboratory practice and the relevant published literature. A number of international publications have described critical value lists. In the early 1990s, Kost[2-3] published surveys of adult and paediatric critical values from medical centres in the USA, and more recently the College of American Pathologists[4] compared the critical values of 163 of its participants. Although these resources are useful for critical value assessment, they may not be clinically relevant to South Africa (SA). Moreover, local lists are not readily available.
A survey was therefore designed to determine the critical value policies for haematology tests in clinical laboratories in SA.
Methods
Study design
A survey form was sent to 136 identified laboratories across SA in January 2013. Of these, 36 (26.5%) responded. Critical values were defined as results requiring urgent notification. Participating laboratories were asked to provide high and low critical values for haematology tests for adults and children and to document their critical value reporting policy. The results were reviewed against published international studies comparing critical values across a large number of laboratories.
Statistical analysis
The survey findings were recorded on an Excel spreadsheet. The mean, standard deviation and range were calculated for each test.
Results
Data were collected from 36 laboratories, of which 11.1% (n=4) were private, 33.3% (n=12) were affiliated to academic institutions, and 55.6% (n=20) were peripheral or regional National Health Laboratory Service (NHLS) laboratories.
Critical value policies
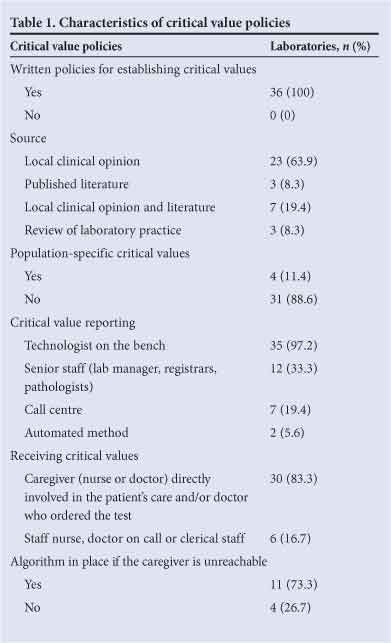
Table 1 summarises the critical value policies of the laboratories surveyed. All confirmed that they had a critical value standard operating policy based on the following sources: local clinical opinion (63.9%, n=23); the published literature (8.3%, n=3); local opinion and the published literature (19.4%, n=7); and review of laboratory practice (8.3%, n=3).
Critical value reporting
Of the laboratories, 97.2% (n=35) indicated that reporting was commonly undertaken by the technologist on duty. In addition, 19.4% of the laboratories (n=7) authorised reporting by a call centre. All laboratories reported critical results telephonically as well as via an automated method such as a fax, e-mail or sms (5.6%, n=2). The majority of the laboratories required that the person notified of the critical value be the doctor or nurse directly involved in the patient's care, and/or the person who ordered the test (83.3%, n=30). A minority of laboratories (16.7%, n=6) permitted reporting of critical values to any nurse, doctor on call or clerical staff member (or did not specify).
Of the 15 laboratories that responded to this question, 73.3% (n=11) reported following an algorithm in the event that the caregiver could not be reached. This resulted in additional time spent. The steps in the algorithms included contacting the departmental head or hospital superintendent (18.1%, n=2) or the doctor on call (45.5%, n=5), delivering the critical values via messenger or automated message (18.1%, n=2), or phoning at least three times (18.1%, n=2).
Critical values for haematology tests
Mean low and high critical limits for the most frequently listed tests were as follows: haemoglobin <6 and >20 g/dl, platelet count <41 and >1 000x109/l, white cell count <2 and >46x109/l, activated partial thromboplastin time (aPTT) >101 seconds, and international normalised ratio (INR) >6 (Table 2). Laboratories also included presence of malaria (72.2%, n=26), haemolysis (16.7%, n=6) and/ or primitive cells/blasts (30.6%, n=11) in their critical value lists on peripheral blood review. A minority of laboratories reported additional critical values for coagulation assays, namely fibrinogen (13.9%, n=5), d-dimer (13.9%, n=5), coagulation factors (2.8%, n=1) and anti-Xa (2.8%, n=1). Specific critical limits for children and neonates were reported by 5.6% (n=2) and 13.9% (n=5) of laboratories, respectively.
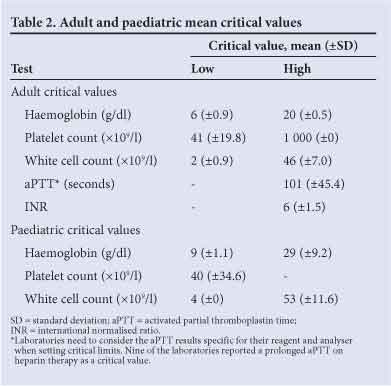
In addition, 5.6% of laboratories (n=2) reported paediatric critical values that were similar to their adult values.
Table 3 shows the comparison of the mean, low and high critical limits of the three laboratory categories. The private laboratories reported a stricter cut-off for the mean lower limit for haemoglobin and platelet count, and for the upper limit for INR compared with the academic and peripheral and regional NHLS laboratories. For example, the mean lower limit for haemoglobin was 6.8 g/dl for private laboratories, 6.0 g/dl for laboratories affiliated to academic institutions, and 5.0 g/dl for peripheral and regional NHLS laboratories. Similarly, the mean upper limit for the INR was 4.4 for private laboratories and 6 for the laboratories affiliated to academic institutions and the peripheral and regional NHLS laboratories.
Discussion
The national focus is currently on improving laboratory commitment to service, efficiency and patient safety. Critical value reporting is an important laboratory quality indicator. The laboratories surveyed indicated that policies were in place for reporting critical values in accordance with accreditation requirements. In the absence of local published literature, the majority of laboratories have relied on local clinical opinions to develop critical value limits. The findings of this survey may be of value to local laboratories that are in the process of establishing or reviewing their critical values policies.
All laboratories surveyed reported adult critical values for the commonly performed haematology tests, namely haemoglobin, platelet count, white cell count, INR and aPTT, that may reflect life-threatening emergencies. The ranges for the critical values reported were very wide. For example, the lower limits for haemoglobin and platelet count ranged from 4 to 8 g/dl and from 10 to 100x109/l, respectively. Laboratories may wish to review their given critical value(s) for clinical relevance in their specific settings.
The variability of the mean critical values between the three laboratory categories is shown in Table 3. The private laboratories reported a stricter cut-off for the mean lower limit for haemoglobin and platelet count and the mean upper limit for INR compared with the academic, peripheral and regional NHLS laboratories. International surveys report similar cut-offs to those reported by the private laboratories. Kost[2] and Wagar et al.[4] reported mean lower limits for haemoglobin of 6.6 and 6.9 g/dl, respectively, as well as a mean upper limit for the INR of 4.0.
In addition to being developed in conjunction with the published literature, it is important that critical value policies are reviewed by local clinicians to ensure that cut-off values are clinically relevant. As seen in the landmark Transfusion Requirements in Critical Care trial,[5] a restrictive transfusion trigger of <7 g/dl for younger (<55 years) and less ill (APACHE II score <20) patients is a clinically relevant limit. Clinical trials, however, show a wide variation in triggers for transfusion.[6] Similarly, an INR of >5 is a clinically relevant limit because it is associated with an increased risk of bleeding.[7] An INR >5 requires urgent intervention, such as withholding warfarin doses or administering specific treatment, such as vitamin K, fresh-frozen plasma or prothrombin complex concentrates, to reverse the effect of warfarin.'71 This serves as a further example of the important role clinicians play in developing their local laboratory's critical value policies.
Critical value policies require notification of the abnormal result to someone who can react appropriately. A minority of laboratories allowed reporting of critical values to someone other than the direct caregiver, e.g. a nurse, a doctor on call or a member of the clerical staff, with the risk that the recipient may know little about the patient's clinical condition and of the clinical significance of the critical result. The majority, however, required that the person notified of the critical value be the doctor who ordered the test or the caregiver directly involved in the patient's care, as is recommended in the literature.[8] Furthermore, significantly, the laboratory staff member can interpret the result with the direct caregiver, and suggest further investigations.[9]
The reporting of critical values generates a significant workload.'101 Published studies report that 1 - 15% of coagulation tests are critical values requiring urgent notification.[11] Laboratories may therefore need to rely on call centres to perform these administrative functions in the future.
Conclusion
Each laboratory is responsible for establishing clinically relevant critical limits and ensuring that the appropriate caregivers are rapidly notified, so that quality patient care can be administered. Clinicians should be involved in developing the laboratory's critical value policy. Critical value calls are costly for laboratories, as extensive time is required by skilled staff to handle reporting. Automated notification systems may need to be considered and investigated.
References
1. Lundberg GD. When to panic over an abnormal value. MLO Med Lab Obs 1972;4:47-54. [ Links ]
2. Kost GJ. Critical limits for urgent clinician notification at US medical centers. JAMA 1990;263(5):704-707. [http://dx.doi.org/10.1001/jama.263.5.704] [ Links ]
3. Kost GJ. Critical limits for emergency clinician notification at United States children's hospitals. Pediatrics 1991;88(3):597-603. [ Links ]
4. Wagar EA, Friedberg RC, Souers R, Stankovic AK. Critical values comparison: A College of American Pathologists Q-Probes survey of 163 clinical laboratories. Arch Pathol Lab Med 2007;131(12):1769-1775. [ Links ]
5. Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999;340(6):409-417. [http://dx.doi.org/10.1056/NEJM199902113400601] [ Links ]
6. Hebert PC, Wells G, Martin C, et al. A Canadian survey of transfusion practices in critically ill patients. Transfusion Requirements in Critical Care Investigators and the Canadian Critical Care Trials Group. Crit Care Med 1998;26(3):482-487. [http://di.doi.org/10.1097/00003246-199803000-00019] [ Links ]
7. Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th ed.). Chest 2008;133(6 suppl):160S-198S. [http://di.doi.org/10.1378/chest.08-0670] [ Links ]
8. Genzen JR, Tormey CA. Pathology consultation on reporting of critical values. Am J Clin Pathol 2011;135(4):505-513. [http://di.doi.org/10.1309/AJCP9IZT7BMBCJRS] [ Links ]
9. Howanitz PJ, Steindel SJ, Heard NV. Laboratory critical values policies and procedures: A college of American Pathologists Q-Probes Study in 623 institutions. Arch Pathol Lab Med 2002;126(6):663-669. [ Links ]
10. Dighe AS, Rao A, Coakley AB, Lewandrowski KB. Analysis of laboratory critical value reporting at a large academic medical center. Am J Clin Pathol 2006;125(5):758-764. [http://dx.doi.org/10.1309/R53XVC2U5CH6TNG8] [ Links ]
11. Pai M, Moffat KA, Plumhoff E, Hayward CP. Critical values in the coagulation laboratory: Results of a survey of the North American Specialized Coagulation Laboratory Association. Am J Clin Pathol 2011;136(6):836-841. [http://dx.doi.org/10.1309/AJCP8O8GIPPPNUSH] [ Links ]
 Correspondence:
Correspondence:
E Schapkaitz
(elise.schapkaitz@nhls.ac.za)
Accepted 27 May 2013














