Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.104 n.1 Pretoria Jan. 2014
RESEARCH
The use of the full blood count and differential parameters to assess immune activation levels in asymptomatic, untreated HIV infection
N VankerI; H IppII
IMB ChB. Division of Haematology, Department of Pathology, National Health Laboratory Service and Stellenbosch University, Tygerberg Hospital, Cape Town, South Africa
IIMB ChB, FCPath (SA)(Haematology). Division of Haematology, Department of Pathology, National Health Laboratory Service and Stellenbosch University, Tygerberg Hospital, Cape Town, South Africa
ABSTRACT
BACKGROUND: A feature of HIV/AIDS is chronic immune activation, which results in a number of complications including inflammation-related disorders and blood cytopaenias. Immune activation status is not routinely tested in HIV infection. However, the full blood count (FBC) is a commonly performed test.
OBJECTIVE: We hypothesised that FBC parameters would be significantly different in HIV-infected v. -uninfected individuals, and that some of these parameters would correlate with markers of immune activation (i.e. percentage CD38 expression on CD8+ T cells (%CD38onCD8)) and disease progression (i.e. CD4+ counts) in HIV infection.
METHODS: This was a cross-sectional study with 83 HIV-infected adults who were antiretroviral therapy-naive and clinically well, and 51 HIV-uninfected adults. The %CD38onCD8 and CD4+ counts were determined by flow cytometry and the FBC was performed on a Siemens ADVIA 2120 system. FBC parameters investigated were total white cell count (WCC), haemoglobin (Hb) concentration, platelet count, absolute neutrophil count, absolute lymphocyte count, and percentage of large unstained cells (%LUCs).
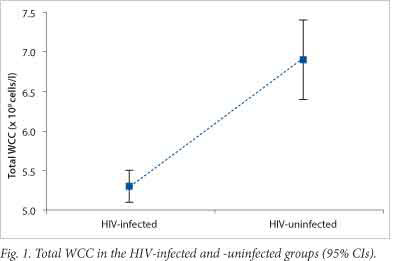
RESULTS: Significant differences were found between the HIV-infected and -uninfected groups for total WCC, Hb, neutrophil count, lymphocyte count and %LUCs. The mean ± standard deviation (SD) for the total WCC (5.3±1.3 v. 6.9±2.2; p<0.001) and the %LUCs (2.5±0.9 v. 2.0±0.9; p=0.001) both showed correlations with CD4+ counts and %CD38onCD8.
CONCLUSION: The total WCC and %LUCs showed significant differences in HIV-infected individuals and correlated with markers of immune activation and disease progression. This suggests the potential use of these parameters as markers of immune activation in HIV infection.
HIV invades the human host and replicates preferentially in CD4+ T cells, leading to increasing viral numbers and a failing immune system. An important feature in the pathogenesis of AIDS is chronic immune activation and inflammation.[1-4] Sustained immune activation creates a suitable environment for viral replication, drives the loss of CD4+ T cells and also leads to inflammation-related disorders such as atherosclerosis, neurocognitive deterioration, musculoskeletal abnormalities and certain cancers.[1-3]
Another complication related to immune activation in HIV is the development of peripheral blood cytopaenias. Cytopaenias are due, in part, to the stimulated immune system releasing pro-inflammatory cytokines, which suppress haemopoiesis in the bone marrow. Other contributing factors to low cell counts include the suppression of bone marrow progenitors by HIV-infected T cells, the myelosuppressive effect of certain drugs, infective and malignant bone marrow infiltration, and immune-mediated peripheral destruction of blood cells.[5-7]
Antiretroviral therapy (ART) is the most effective treatment for patients with HIV/AIDS.[1,3,5] The current criteria for initiating ART are based on either the presence of an AIDS-defining illness, the quantification of CD4+ T cells (or total lymphocyte counts, in situations where CD4+ counts are not available) or a combination of clinical HIV disease staging and CD4+ (or lymphocyte) counts.[5] Once ART is initiated, patients are followed-up at regular intervals with clinical examinations, CD4+ counts and viral-load testing.[5]
Although the degree of immunodeficiency and viral burden are monitored routinely, immune activation status is not currently tested in HIV infection.[2,4,5] Many serum markers of inflammation or immune stimulation in HIV infection have been identified, including the well-established cellular marker of immune activation, CD8+ T cells that express the activation marker CD38.[1,2,4] CD38 expression on CD8+ cells (CD38onCD8) is an important prognostic marker of HIV disease progression, independent of immunodeficiency and viral burden.[1,4] However, determination of CD38onCD8 expression by flow cytometry is an expensive and technically difficult test to perform in resource-limited settings.[4,8]
In 2002, a working group was established to develop an approach to clinical research priorities for ART use in resource-limited settings. Issues addressed included the timing of initiation of therapy, choice of ART agents, adherence to therapy and the monitoring of patients receiving treatment. With regard to the latter, the development of simpler, more cost-effective tests, which could assess viral burden and immune function, to replace viral loads and CD4+ counts was recommended.[9]
For HIV-infected patients, either prior to or after initiation of ART, a full blood count (FBC) and differential count are regularly requested owing to the prevalence of cytopaenias, opportunistic infections and adverse drug events.[5]- The cytopaenias include anaemia, leucopaenia, neutropaenia, lymphopaenia and thrombocytopaenia, which may exist as isolated features, or in various combinations. [6] Parameters routinely included in an FBC are the total white cell count (WCC), haemoglobin (Hb) concentration and a platelet count as well as a leucocyte differential count. The leucocyte differential count includes the neutrophil, lymphocyte, monocyte, eosinophil and basophil counts. On certain haematology analysers, (i.e. the Siemens ADVIA 2120) the percentage of large unstained cells (%LUCs) also forms part of the differential count. LUCs usually encompass virally activated lymphocytes, plasma cells, hairy cells, paediatric lymphocytes and peroxidase-negative blasts.[10] The FBC is an affordable test available in most intermediate (level 2) and central (level 3) laboratories.[5] Furthermore, it is recommended that if CD4+ counts are not available, total lymphocyte counts, performed as part of the differential count on routine haematology analysers[5,10] may be seen as a surrogate marker of immune function.
Objective
To determine whether certain FBC parameters are significantly different in HIV-infected patients v. uninfected individuals, and whether these parameters correlate with markers of HIV disease progression and immune activation in an untreated local population group. We hypothesised that the WCC, platelet count and Hb concentration would be lower in the HIV-infected cohort, and that the %LUCs would be raised. We further hypothesised that these FBC parameters would correlate with CD4+ counts, viral loads and %CD38onCD8. A secondary objective of the study was to determine whether testing of FBC parameters would be more affordable than %CD38onCD8 determination.
Methods
Study design
This was a cross-sectional study of 134 adults recruited from a single HIV counselling and testing (HCT) clinic in Crossroads, Cape Town, South Africa. The prevention clinic employs the national HCT algorithm with accredited rapid tests. Study participants were recruited between May 2010 and June 2012 and were enrolled in the study after confirmation of HIV status and before referral for further management. Exclusion criteria were: individuals <21 years of age, patients receiving ART, features of tuberculosis (TB) or individuals receiving anti-TB therapy or other medications, and symptoms of systemic illness (as assessed by a clinic nurse). Peripheral blood was drawn from all patients and used to measure the FBC and differential count, %CD38onCD8, the CD4+ count and the viral load (the latter tested in the HIV-infected study participants only).
The study was approved by the Stellenbosch University Human Research Ethics Committee (ref no. N07/09/197) and the University of Cape Town Research Ethics Committee (ref no. 417/2006), and performed in accordance with the Declaration of Helsinki.
Whole blood analysis
Whole blood was analysed on a Siemens ADVIA 2120 system, which makes use of cytochemistry and flow cytometry systems. Hb was measured by modification of the cyanmethaemoglobin method together with a cell-based method. Platelets were analysed using two angles of light scatter, used to calculate the volume and refractive index. A combination of cell size, myeloperoxidase staining and nuclear lobulation was used to perform the total leucocyte and 6-part differential count. We assessed the following parameters: the total WCC, absolute neutrophil and absolute lymphocyte count, and %LUCs. LUCs are peroxidase-negative cells, which do not fit into the other categories of leucocytes, i.e. neutrophils, monocytes, eosinophils, lymphocytes and basophils.
The %CD38onCD8 was determined by flow cytometry. Heparinised whole blood was processed on the same day as the sample was drawn. A total of 50 µl of whole blood was incubated with the following monoclonal antibodies: CD8-PerCP; CD38-APC; CD3-FITC (BD Biosciences) for 20 minutes at room temperature. The red blood cells were lysed in BD FACS Lyse and the remaining cells washed with staining buffer (2% fetal calf serum in phosphate-buffered saline) prior to acquisition and analysis on a BD FACSCalibur instrument using BD Cell Quest Pro (version 2) software. Lymphocytes were gated on forward v. side scatter and CD3 expression, and %CD38onCD8 within the lymphocyte gate was determined.
Flow cytometry for CD4+ T cell counts was performed using whole blood. The BD MultiTEST CD3-FITC/CD8-PE/CD45-PerCP/CD4-APC reagent together with TruCOUNT tubes (BD Biosciences) was used for determining CD4+ counts.
HIV-1 RNA quantifications for viral load testing were performed using 1 ml of plasma with the Nuclisens Easy Q HIV-1 v.1.2 kit (BioMerieux Inc), an assay with a lower detection limit of 40 - 50 copies/ml.
Statistical analysis
The Mann-Whitney U test was used to determine differences between the means, standard deviations and 95% confidence intervals (CIs). The Spearman rank order method was used to identify correlations between the FBC parameters and the established markers, which were %CD38onCD8, CD4+ count and viral load.
Costing
Although a complete cost analysis was not carried out, we compared the pricing of these tests based on how much it would cost the consumer in our setting. In SA, state-funded laboratory testing is performed by the National Health Laboratory Service (NHLS); costs quoted below were taken from the Department of Health website.[8]
Results
The 83 HIV-infected individuals were naive to ART, not receiving anti-TB or other medication and clinically asymptomatic. Their median age was 32 years and there was a female to male ratio of 2.8:1. The 51 HIV-uninfected individuals were from the same population, with a median age of 31.8 years and a female to male ratio of 2:1.
Statistically significant differences were found between the HIV-infected and -uninfected groups for %CD38onCD8, CD4+ counts, total WCCs, Hb concentrations, absolute neutrophil counts, absolute lymphocyte counts and %LUCs (Table 1). The 95% CIs for total WCC and %LUCs are shown in Figs 1 and 2, which demonstrate the significant differences, with no overlapping ranges. No statistical difference was observed between the HIV-infected and -uninfected groups for the platelet count (Table 1). There were significant positive correlations between %CD38onCD8 and both %LUCs and total WCC, and there was a significant inverse correlation between %CD38onCD8 and Hb concentration. There were also significant positive correlations between CD4+ counts and the absolute lymphocyte count, the absolute neutrophil count and the total WCC, and an inverse correlation between the CD4+ count and %LUCs (Table 2).

Although the %CD38onCD8 is not a routinely performed test, it is possible to request a flow cytometry-based analysis, which would cost R629.40 (~64 USD), based on the number of antibodies used.[8] The FBC costs R50.25 (~5 USD) and the leucocyte differential costs R27.55 (~3 USD).[8] The total cost of a full blood and differential count is R77.80 (~8 USD).[8]
Discussion
Immune activation is an important prognostic factor in HIV infection.[1-4] Affordable markers of immune activation would be of value in the management algorithms of early HIV infection in resource-limited settings. In the current study, of the parameters assessed on the FBC, the total WCC and %LUCs demonstrated good potential for future use in this context. The total WCC was significantly lower in the HIV-infected group and the %LUCs were significantly higher than in the uninfected group, with both markers showing clearly defined 95% CIs (Figs 1 and 2). Furthermore, both parameters correlated with the CD4+ count (an indicator of HIV disease progression) and %CD38onCD8 (an established marker of immune activation in HIV infection).
Leucopaenia in HIV infection is thought to be predominantly due to lymphopaenia and neutropaenia.[6,11] Studies have shown that lymphopaenia and depletion in lymphocyte subsets correlate with markers of immune activation in HIV infection.[11,12] However, total WCC has not been previously studied as a potential marker of generalised immune activation levels in untreated HIV-infection. In this study, we show for the first time that the lower the total WCC, the higher the levels of immune activation (%CD38onCD8). This is likely to be due to a combination of factors, including the pro-inflammatory environment in HIV infection, which primes activated cells for accelerated death,[1] and suppresses the regeneration of the immune system in the bone marrow, thymus and lymph nodes.[1,6]
Very few studies have focused on the clinical implications of LUCs in humans, most assessing LUCs in the context of haematological malignancies and not immune activation.[13,14] A study from Europe reviewed FBC reports to identify and describe cases with raised LUCs. The study found that in the very small percentage (0.007%) of reports that identified high numbers of LUCs, the majority of cases (85%) had associated viral infections (i.e. Epstein-Barr virus and influenza A) while the remaining 15% were associated with chronic renal failure. The HIV status of the patients was not reported. It was suggested that high numbers of LUCs, where chronic renal failure and acute leukaemia have been excluded as a cause, represent natural killer cells, cytotoxic lymphocytes or other reactive lymphoid cells. [15] This suggests that LUCs represent virally activated lymphocytes, consistent with our hypothesis that circulating LUCs would be present in higher numbers in individuals with chronic immune stimulation.
Our study found significantly lower neutrophil and lymphocyte counts and Hb concentrations in the HIV-infected group. Lower blood cell counts/cytopaenias are a common effect of HIV infection and mechanisms include decreased bone marrow production and increased peripheral destruction of cells. Causes are multifactorial, contributing factors include immune activation and release of cytokines, effects of ART and opportunistic infections.[6] As all individuals in our study were ART-naive and did not show clinical features of opportunistic infections, their cytopaenias were most likely due to cytokine-induced myelosuppression, or enhanced destruction due to immune mechanisms. The significantly raised %CD38onCD8 found in these individuals adds support to the concept of ongoing immune activation as contributing to the cytopaenias.
Some of our findings were comparable with those in a study conducted in an HIV-infected, predominantly adult population to assess the presence of cytopaenias in relation to CD4+ counts. This study found that lower CD4+ counts (<200 cells/µl) were associated with a higher prevalence of anaemia, neutropaenia and thrombocytopaenia.'[7] Our study also found declining CD4+ counts associated with lower Hb concentrations and neutrophil counts but there were no significant findings in relation to platelet counts. Another comparative study, conducted among HIV-positive, ART- naive adolescents, found lower WCCs, neutrophil counts and CD4+ counts, as well as higher expression of %CD38onCD8.[11]
There was a significant positive correlation between both the %LUCs and the total WCC, and %CD38onCD8, the established marker of immune activation.
The total cost of a full blood and differential count is R77.80 (~8 USD), compared with the %CD38onCD8 price of R629.40 (~64 USD), the blood parameters costing approximately eight times less.[8] Using total WCC or %LUCs, either independently or together, could serve as relatively inexpensive markers of immune activation in HIV infection.
The clinical consequences of chronic immune activation are inflammation-related conditions, including cardiovascular, neurological, musculoskeletal and malignant diseases.[1-3] In addition, studies have shown that patients receiving ART and with suppressed viral loads remain at risk of inflammation-related morbidity, owing to parameters other than viral burden, which drive immune activation. Clinical trials are being carried out using adjusted ART regimens and anti-inflammatory agents, in an effort to boost CD4+ counts and decrease systemic immune activation.[2,3] The clinical utility of immune activation markers, such as total WCC and %LUCs, would be to identify HIV-infected individuals who are at a greater risk of inflammation-related co-morbidities. These individuals would then benefit from further investigation for underlying inflammatory conditions and appropriate management.
A possible limitation of our study was its cross-sectional nature, which provided a 'snap-shot' insight into immune activation levels. In addition, specific investigations for other underlying co-infections were not performed. Longitudinal studies will be of value to determine the predictive power of lower WCCs and raised LUC levels for disease progression, development of complications or response to various treatment interventions.
Conclusion
The total WCC was significantly decreased and %LUCs significantly increased in the HIV-infected study population, substantiating the relevance of these markers in HIV infection. The correlations observed between both total WCC and %LUCs and the CD4+ count, support the potential use of total WCC and %LUCs as markers of HIV disease progression. Furthermore, there were significant correlations between both total WCC and %LUCs and the %CD38onCD8, suggesting that these FBC parameters may serve as useful markers of immune activation. The FBC and differential count are affordable, routinely performed tests, even in resource-limited settings. Intervention studies will be important to determine whether these FBC parameters are surrogate markers of immune activation in HIV infection.
Acknowledgements. This research was supported by the following organisations: the NHLS-K, the Poliomyelitis Research Foundation and the South African HIV/AIDS Research and Innovation Platform. We thank the patients and staff of the Emavundleni Clinic in Crossroads, Cape Town for their participation in this study. We also thank other members of the HIV Activation and Inflammation Group (HAIG) for their assistance with specimen processing and running of tests.
References
1. Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: Causes and consequences. J Pathol 2008;214(2):231-241. [http://dx.doi.org/10.1002/path.2276]
2. Nixon DE, Landay B. Biomarkers of immune dysfunction in HIV. Curr Opin HIV AIDS 2010;5(6):498-503. [http://dx.doi.org/10.1097/COH.0b013e32833ed6f4] [ Links ]
3. Deeks SG. Immune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapy. Top HIV Med 2009;17(4):118-123. [ Links ]
4. Liu Z, Cumberland WG, Hultin LE, et al. CD8+ T-lymphocyte activation in HIV-1 disease reflects an aspect of pathogenesis distinct from viral burden and immunodeficiency. J Acquir Immune Defic Syndr Hum Retrovirol 1988;18(4):332-340. [http://dx.doi.org/10.1097/00042560-199808010-00004] [ Links ]
5. World Health Organization. Guidelines for HIV Diagnosis and Monitoring of Antiretroviral Therapy. New Delhi: WHO. 2005. http://www.aidsdatahub.org/dmdocuments/Guidelines_for_HIV_Diagnosis_ and_Monitoring_of_Antiretroviral_Therapy_2005.pdf.pdf (accessed 22 July 2013). [ Links ]
6. Opie J. Haematological complications of HIV infection. S Afr Med J 2012;102(6):465-468. [ Links ]
7. De Santis GC, Brunetta DM, Vilar FC, et al. Hematological abnormalities in HIV-infected patients. Int J Infect Dis 2011;15(12):e808-e811 [http://dx.doi.org/10.1016/jijid.201L08.001] [ Links ]
8. National Health Laboratory Service. State Pricing Catalogue 2011/2012. Johannesburg: NHLS, 2012. http://www.doh.gov.za/docs/programmes/2012/appendixM.pdf (accessed 13 November 2012). [ Links ]
9. Rabkin M, El-Sadr W, Katzenstein D, et al. Antiretroviral treatment in resource-poor settings: Clinical research priorities. The Lancet 2002;360(9344):1503-1505. [http://dx.doi.org/10.1016/S0140-6736(02)11478-4] [ Links ]
10. Buttarello M, Plebani M. Automated blood cell counts: State of the art. Am J Clin Pathol 2008;130:104-116. [http://dx.doi.org/10.1309/EK3C7CTDKNVPXVTN] [ Links ]
11. Douglas SD, Rudy B, Muenz L, et al. Peripheral blood mononuclear cell markers in antiretroviral therapy-naive HIV-infected and high risk seronegative adolescents. AIDS 1999;13(13):1629-1635. [http://dx.doi.org/10.1097/00002030-199909100-00005] [ Links ]
12. Sousa AE, Carneiro J, Meier-Schellershein M, Grossman Z, Victorina RMM. CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV-1 and HIV-2 but only indirectly to the viral load. J Immun 2001;169:3400-3406. [ Links ]
13. Lilliehook I, Tvedten HW. Errors in basophil enumeration with 3 veterinary hematology systems and observations on occurrence of basophils in dogs. Vet Clin Pathol 2011;40(4):450-458. [http://dx.doi.org/10.1111/j.1939-165X.2011.00353.x] [ Links ]
14. Bononi A, Lanza F, Ferrari L, et al. Predictive value of hematological and phenotypical parameters on postchemotherapy leukocyte recovery. Cytometry B: Clin Cytom 2009;76(5):328-333. [http://dx.doi.org/10.1002/cyto.b.20476] [ Links ]
15. Nixon DF, Parsons J, Eglin RP. Routine full blood counts as indicators of acute viral infections. J Clin Pathol 1987;40:673-675. [http://dx.doi.org/10.1136/jcp.40.6.673] [ Links ]
 Correspondence:
Correspondence:
N Vanker
(dr.n.vanker@gmail.com)
Accepted 11 June 2013














