Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.103 no.12 Pretoria Dez. 2013
RESEARCH
A follow-up cross-sectional study of environmental lead exposure in early childhood in urban South Africa
N NaickerI, II; A MatheeIII, IV, V; B BarnesVI
IMB BCh, MMed (Com Health), FCPHM, PhD.South African Medical Research Council, Johannesburg, South Africa
IIMB BCh, MMed (Com Health), FCPHM, PhD.School of Public Health, University of the Witwatersrand, Johannesburg, South Africa
IIIPhD. outh African Medical Research Council, Johannesburg, South Africa
IVPhD. School of Public Health, University of the Witwatersrand, Johannesburg, South Africa
VPhD. Faculty of Health Sciences, University of Johannesburg, South Africa
VIPhD. School of Human and Community Development, University of the Witwatersrand, South Africa
ABSTRACT
BACKGROUND: Lead exposure has significant detrimental effects on the health and wellbeing of children. In resource-poor countries, information on the extent of lead exposure is often inadequate owing to the lack of surveillance and screening programmes.
OBJECTIVE: To determine the degree of lead exposure in children residing in South African urban areas.
METHODS: A cross-sectional survey was conducted in schools in Johannesburg, Cape Town and Kimberley in 2007 - 2008. Blood lead levels were assessed in a total of 1 349 grade 1 children using the LeadCare Analyser system. Parents completed a structured questionnaire on sociodemographic profiles and risk factors to provide information about socioeconomic status and other risk factors for lead exposure.
RESULTS: Blood lead levels ranged from 0.8 - 32.3 µg/dl. The mean blood lead level in the total sample was 7.97 µg/dl; 74% had blood lead levels >5 µg/dl. The highest proportion (84%) of children with blood lead levels >5 µg/dl was in Johannesburg. In the multivariate analysis, socioeconomic status was significantly associated with blood lead levels >5 µg/dl.
CONCLUSION: Lead exposure in South African urban areas remains widespread. The risk of lead poisoning in some areas and certain groups of children may be increasing despite the phasing out of lead-containing petrol. Children living in poverty continue to be the most vulnerable.
Exposure to environmental lead continues to be a major public health challenge, despite progress made by governments and international organisations to eliminate some of the most important sources of exposure, such as petrol and paint. In South Africa (SA), incremental reductions in the maximum lead content of petrol commenced in 1986, with the use of leaded petrol discontinued in 2006.[1] In 2009, legislation was passed banning the use of lead in paint used in homes and for children's toys and furniture.[2] However, the use of lead continues in many other products and services, e.g. in the mining, fishing and hunting sectors, as well as in numerous cottage industries.[3] As a result, soil and dust around homes and schools may have elevated lead loads from historical and various current sources.[3,4]
In Cape Town, SA, cross-sectional studies have shown a decline in blood lead levels (BLLs) among 7 - 11-year-olds from a mean of 11.9 µg/dl in 1991 to 9.1 µg/dl in 2002. Approximately 10% of children had BLLs >10 µg/dl in 2002.[5] In Mumbai, India, after the phasing out of leaded petrol, the proportion of children with very high BLLs (>10 µg/dl) dropped from 60% in 1997 to 37% in 2002. [6] However, a recent study of children aged 6 - 7 years in Chennai, India, showed that 54.5% had BLLs ≥10 µg/dl, with a mean BLL of 11 µg/dl.[7] BLLs in developing countries are markedly higher than international guidelines permit.[8] In addition, because of local lead sources, there may be areas and child groups with BLLs ≥10 µg/dl. In resource-rich countries such as the USA, the average BLL for children is around 3 µg/dl, which is a significant decrease from levels found in the 1970s.[9]
Nevertheless, BLLs as low as 3 µg/dl have been associated with adverse cognitive and behavioural outcomes in children.[7,10-12] Widespread exposure to low levels of environmental lead may have a large public health impact, especially in resource-poor countries such as SA where resources to mitigate the adverse effects are limited, and where there are no blood lead surveillance and screening programmes.
Objectives
To assess BLLs of grade 1 children attending schools in the cities of Cape Town, Johannesburg and Kimberley, and to explore risk factors for increased levels of exposure. This study is a follow-up of previous cross-sectional surveys conducted in 1995 and 2002 in the same schools.
Methods
Study population
A cross-sectional, analytical study was undertaken between 2007 and 2008 at primary schools in the cities of Cape Town, Johannesburg and Kimberley. These were the same towns and schools included in previous such studies.[1,5,13] Cape Town, the largest coastal city in Western Cape Province, covers ~2 400 km2 with a population of ~3.4 million in 2007. Johannesburg, in Gauteng Province, covers ~2 300 km2 with a population of 3.8 million; it is one of the fastest growing cities in the world. Kimberley, until 1914 a diamond mining town, is the capital of Northern Cape Province, with a population of ~200 000. Schools were selected to reflect lead exposure in children spending time close to, or distant from, heavily trafficked roads. Children in grade 1 were included in the study after obtaining written informed consent from their parents or guardians, and assent from the children themselves on the day of the study.
Data collection
Structured questionnaires were designed to obtain information about the health of the study children, household socioeconomic status, housing type and other potential risk factors for elevated BLLs. Questionnaires were available in English and Afrikaans, which were the predominant languages spoken in the selected towns. The translation of questionnaires was by professional translators and back-translated for accuracy. Questionnaires were distributed by the schools to the homes of study children in advance, and self-administered by parents or guardians. Parents were requested to return completed questionnaires and consent forms to the school on the following day. Respondents were provided with contact details for the research team, and were encouraged to make contact in the event of any uncertainty about the questionnaire.
On the day of data collection, height and weight measurements were taken of each child. Finger-prick blood tests were obtained for BLLs using the LeadCare 1 Analyser system (ESA Biosciences, USA). Whole blood from the capillary tube was decanted into the LeadCare containers containing reagent. After 1 minute, a 50 µl pipette was used to transfer the blood/reagent mixture onto a sensor strip. The sensor was inserted into the analyser and a result was displayed in 3 min. This system has been used in previous studies and its reliability demonstrated.[14] Disposable sterile blood sampling equipment and aseptic sampling techniques were used throughout.
Analysis
Participants' BLLs were the dependent (outcome) variable. BLLs were not normally distributed but skewed positively and thus were log transformed; however, this did not change the findings. BLLs were analysed as a continuous variable in the bivariate analysis using Student's t-test and then stratified into low (<5 µg/dl) and high (≥5 g/dl) levels to determine odds ratios (ORs) in the bivariate and multivariate analysis. The US Centers for Disease Control (CDC) has recently recommended a new reference level based on the US population of children aged 1 - 5 years who are in the top 2.5% of the national blood lead distribution. Currently, this level is 5 (µg/dl, and was used to dichotomise the blood lead distribution in the current study.[8] Predictor or risk factor variables included sex of the child, socioeconomic factors, housing type and conditions, and behaviour of any child or adult in the household that might lead to increased exposure to lead. A p-value <0.05 was considered significant. All factors that were significantly associated with high BLLs were included in the multivariate analysis. Data were analysed using Stata 12 statistical software.
Ethics
Ethical approval for the study was secured from the Human Research Ethics Committee of the University of the Witwatersrand in Johannesburg. Written consent forms were completed and signed by parents of the children prior to data collection and blood sampling. Parents were informed that participation was voluntary, and of their right to withdraw at any time without detrimental consequences. The ethics approval number for this study was M070458.
Results
Sample characteristics
A total of 1 349 grade 1 children (51% boys) were included; 36% (479) were from Cape Town, 52% (704) from Johannesburg and 12% (166) from Kimberley. The mean age was 7.62 years (ranged 5 - 12 years). About 96% were SA citizens.
Most (33%) households earned <R1 000 (US$ 110) per month. Only 17% of households earned >R5 000 (>$550) per month. Over 91% of households had at least one employed adult. Households consisted of an average of 6 people. Of the total, 77% lived in formal dwellings; 39% of dwellings required major repairs, and 40% reported having peeling paint inside or outside the home. Electricity was the main source of energy for cooking in 90% of homes, and 60% of homes had copper or plastic water pipes. A total of 52% of homes were reported to be situated within one block of a busy road.
Analysis of behavioural risk factors showed that 43% of children lived with an adult who smoked, and that 32% of the sample lived in a household that included a member whose occupation potentially involved lead use, e.g. vehicle repairs, spray painting, building or renovation, painting, welding, or repairing of electrical appliances. A total of 28% of children played with pets, 22% used homemade or traditional remedies, and 9% used make-up such as eyeliner or kohl pencils, which potentially contain lead.
Blood lead results
The mean BLL in the total sample was 7.97 µg/dl (standard deviation (SD) ±4.37), while the median was 7 µg/dl. Individual BLLs ranged from 0.8 to 32.3 µg/dl, and 74% of the sample had BLLs ≥5 µg/dl. The Johannesburg sample had the highest proportion, at 84% of children with BLLs ≥5 µg/dl, while 57% and 66% were similarly affected in Kimberley and in Cape Town, respectively (Table 1).
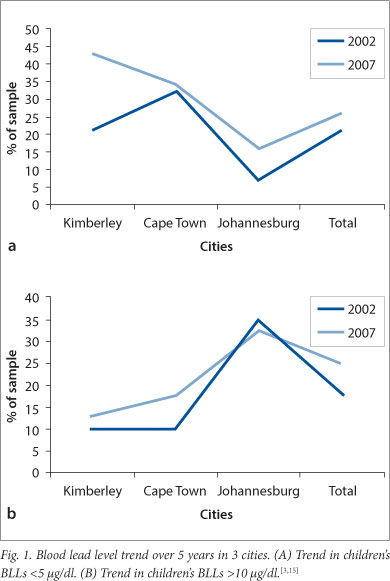
The percentage of children with BLLs <5 µg/dl increased in all three sites from 2002 to 2007. The proportion, however, with BLLs >10 µg/dl increased in Kimberley and Cape Town, although the overall difference was not significant (p=0.26) (Fig. 1).
Risk factors for high BLLs (≥5 |ig/dl)
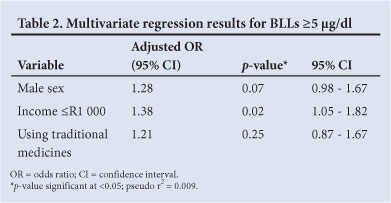
In the bivariate analysis, only 3 variables were significantly associated with an increased risk of exposure: male child, household income <R1 000, and use of traditional medicines. After adjusting for confounding variables in the multivariate analysis, only low income levels were significantly associated with high BLLs, with an OR of 1.4 (Table 2). Being male or using traditional medicines increased the odds of having an elevated BLL (OR >1.2), but the association was not statistically significant.
Discussion
The study demonstrates the extent of lead exposure and poisoning in urban SA children. Lead poisoning is preventable, yet 74% of children had BLLs ≥5 µg/dl (i.e. more than the current reference level of the CDC).[8] The proportions with BLLs ≥5 µg/dl for the Kimberley and Cape Town samples were 57% and 66%, respectively. As many as a quarter of children in the sample had BLLs ≥10 µg/dl (which is the level previously endorsed by the World Health Organization (WHO), but currently under review). Children in Johannesburg were most seriously affected, having the highest proportion with BLLs >10 µg/dl (33%). In the Kimberley and Cape Town samples, 13% and 18% of the sample, respectively, had BLLs ≥10 µg/dl.
The most notable risk factor for lead exposure in this study was the socioeconomic status of the children. Children in households with an income <US$125 per month were significantly more at risk of having BLLs ≥5 µg/dl. Many previous studies have consistently highlighted the role of low socioeconomic status in increasing the risk of lead exposure.[5,16] People in poor communities have a greater likelihood of having jobs involving lead (and a higher risk of para-occupational exposure), a higher level of involvement in cottage industries (potentially leading to lead exposure in the home setting), poorer levels of nutrition that may exacerbate the effects of lead exposure, and fewer resources to prevent lead exposure and mitigate the health effects, especially the sociobehavioural problems, that arise from lead exposure.[17] It bears noting that there is a large, and growing, gap between the rich and poor in SA, which is among the most unequal societies in the world.
In the 2002 survey, 78% of children in the same schools in Johannesburg had BLLs >10 µg/dl.[12] The decline in children with very high BLLs indicates a reduction in exposure that might be attributed to the discontinuation of leaded petrol. Similar findings have been found in other countries.[6,7,9] However, in Cape Town in 2002, 10% of children in the same schools had BLLs >10 µg/dl compared with 18% in 2007,[15] representing an 8% increase in BLLs, which may be due to the residual effects of past usage of leaded petrol, and leaded paint in homes, toys, playground equipment and informal industries.[5,14] It must also be noted that different methods were used to assess BLLs in 2002 compared with the 2007 survey; however, previous studies have found the LeadCare system to be comparable to formal laboratory analytical methods.[4] There may be pockets of children in urban SA that continue to be at risk, despite the removal of major sources of exposure.
Boys were found to have a significant increase in mean BLLs in the 2002 survey.[13] The present study showed that, after adjusting for confounding factors, being male was not a significant risk factor, although the mean BLL was 0.63 µg/dl higher in males than in females. In other resource-poor countries, such as Uganda and India, no association was found between sex and BLLs.[7,18] However, a study conducted in an older cohort of children (13 years of age) in Johannesburg, found that male sex was a significant risk factor - OR 3.01 (95% CI 2.41 - 3.77).[16]
Household factors such as informal housing, housing with chipped or peeling paint or needing repair and positioned near a busy road, and behavioural factors such as smoking, childhood pica and playing with pets did not influence BLLs in this study. Recent publications have highlighted the use of certain types of traditional medicine that has resulted in cases of lead poisoning in children and adults.[19] While 22% of children in this study were reported to take traditional medicine, this proved to be not significant.
Study limitations
There are several limitations to the present study. It is a cross-sectional analysis and therefore measured blood lead at a single point in time. Since blood lead indicates current exposure (1 month), past exposure might have been different. BLLs could also be high owing to release from bone stores at the time of measurement. Unfortunately, owing to financial constraints, concurrent analyses of possible sources of exposure such as soil in homes and schools, paint and dust swabs could not be conducted.
Given this evidence that lead poisoning in SA is a serious and widespread public health problem, it is imperative that the National Department of Health establish a programme on lead poisoning prevention that would include the development of a national surveillance network, identification of high-risk groups, services and products, and introduce screening programmes in high-risk areas and groups.
Conclusion
This study has highlighted that, 1 - 2 years after the discontinuation of leaded petrol, children's BLLs remain unacceptably high and that, in some areas, the proportion of children with very high BLLs (≥10 µg/dl) has been rising. The major contributing factor is childhood poverty.
Author contributions. NN conceived the paper, data management, data analysis and primary write-up and submission of the paper. AM conceived the study and helped to draft the paper. BB participated in the design and data collection and helped to draft the paper.
Acknowledgements. The study was jointly funded by the South African Medical Research Council and the National Department of Minerals and Energy of South Africa. The authors thank Mirriam Mogotsi and Rochelle Spadoni for their significant contributions with data collection.
References
1. Mathee A, Rollin H, von Schirnding Y, Levin J, Naik I. Reductions in blood lead levels among school children following the introduction of unleaded petrol in South Africa. Environ Res 2006;100:319-322. [http://dx.doi.org/10.1016/j.envres.2005.08.001] [ Links ]
2. Acts Online. The Hazardous Substances Act, 1973. Amendment. http://www.acts.co.za/recent_additions_to_acts_online.html (accessed 1 August 2012). [ Links ]
3. Tong S, von Schirnding YE, Prapamontol T. Environmental lead exposures: A public health problem of global dimensions. Bull World Health Organ 2000;78(9):1068-1077. [http://dx.doi.org/10.1590/S0042-96862000000900003] [ Links ]
4. Kootbodien T, Mathee A, Naicker N, Moodley N. Heavy metal contamination in a school vegetable garden in Johannesburg. S Afr Med J 2012;102(4):226-227. [ Links ]
5. Mathee A, von Schirnding Y, Montgomery M, Röllin H. Lead poisoning in South African children: The hazard is at home. Rev Environ Health 2004;19(3-4):347-359. [ Links ]
6. Nichani V, Li WI, Smith MA, Noonan G, Kodavor M, Naeher LP. Blood lead levels in children after phase-out of leaded gasoline in Bombay, India. Sci Tot Environ 2006;363:95-106. [http://dx.doi.org/10.1016/j.scitotenv.2005.06.033] [ Links ]
7. Roy A, Bellinger D, Hu H, et al. Lead exposure and behaviour among young children in Chennai, India. Environ Health Persp 2009;117(10):1607-1611. [http://dx.doi.org/10.1289/ehp.0900625] [ Links ]
8. Centers for Disease Control. CDC response to advisory committee on childhood lead poisoning prevention recommendations. In: Low Level Lead Exposure Harms Children: A Renewed Call for Primary Prevention. Atlanta: CDC, 2012. http://www.cdc.gov/nceh/lead/ACCLPP/CDC_Response_Lead_Exposure_Recs.pdf. (accessed 1 August 2012). [ Links ]
9. Jones RL, Home DM, Meyer PA, et al. Trends in blood lead levels and blood lead testing among US children aged 1-5 years, 1988-2004. Pediatrics 2009;123(3):e376-e385. [http://dx.doi.org/10.1542/peds.2007-3608] [ Links ]
10. Canfield RC, Henderson CR, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 (g/dl. N Engl J Med 2003;348:1517-1526. [http://dx.doi.org/10.1056/NEJMoa022848] [ Links ]
11. Chandramouli K, Steer CD, Ellis M, Emond MA. Effects of early childhood lead exposure on academic performance and behaviour of school age children. Arch Dis Child 2009;94(11):844-848. [http://dx.doi.org/10.1136/adc.2008.149955] [ Links ]
12. Naicker N, Richter L, Mathee A, Becker P, Norris S. Association between environmental lead exposure and socio-behavioural adjustment: Findings from the Birth to Twenty Cohort. Sci Tot Environ 2012;414:120-125. [http://dx.doi.org/10.1016/j.scitotenv.2011.11.013] [ Links ]
13. Mathee A, von Schirnding YER, Levin J, Ismail A, Huntley R, Cantrell A. A survey of blood lead levels among young Johannesburg school children. Environ Res 2002;90:181-184. [ Links ]
14. Al-Jawadi AA, Al-Mola ZWA, Al-Jomard R Determinants of maternal and umbilical blood lead levels: A cross-sectional study, Mosul, Iraq. BMC Research Notes 2009;2:47. [http://dx.doi.org/10.1186/1756-0500-2-47] [ Links ]
15. Naicker N, Mathee A, Barnes B. Environmental lead: A public health challenge in South Africa. Epidemiology 2013;24(4):621-622. [http://dx.doi.org/10.1097/EDE.0b013e318296c077] [ Links ]
16. Naicker N, Norris SA, Mathee A, von Schirnding YE, Richter L. Prenatal and adolescent blood lead levels in South Africa: Child, maternal and household risk factors in the Birth to Twenty Cohort. Environ Res 2010;110:355-362. [http://dx.doi.org/10.1016/j.envres.2010.02.006] [ Links ]
17. Bellinger DC. Lead. Pediatrics 2004;113(4):1016-1022. [ Links ]
18. Graber LK, Asher D, Anandaraja N, et al. Childhood lead exposure after the phaseout of leaded gasoline: An ecological study of school age children in Kampala, Uganda. Environ Health Persp 2010;118(6):884-889. [http://dx.doi.org/10.1289/ehp.0901768] [ Links ]
19. Gunturu KS, Nagarajan P, McPhedran P, et al. Ayurvedic herbal medicine and lead poisoning. J Hematol Oncol 2011;4:51. [http://dx.doi.org/10.1186/1756-8722-4-51] Accepted 18 July 2013.
 Correspondence:
Correspondence:
N Naicker
(nisha.naicker@gmail.com)
Accepted 18 July 2013














