Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.103 no.11 Pretoria Jan. 2013
RESEARCH
Prevalence, risk factors and risk perception of tuberculosis infection among medical students and healthcare workers in Johannesburg, South Africa
A van RieI; K McCarthyII; L ScottIII; A DowI; W D F VenterII; W S StevensIII, IV
IDepartment of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, USA
IIWits Reproductive Health and HIV Institute, University of the Witwatersrand, Johannesburg, South Africa
IIIDepartment of Molecular Medicine and Haematology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IVNational Health Laboratory Service, Johannesburg, South Africa
ABSTRACT
BACKGROUND: Tuberculin skin test (TST) and interferon gamma release assays (IGRAs) are both recommended for routine screening of healthcare workers (HCWs) in low tuberculosis (TB)-burden countries. More recently, based on scarce data, the World Health Organization strongly recommended that IGRA should not be used for occupational screening in high-burden settings.
OBJECTIVE: To assess the prevalence of latent tuberculosis infection (LTBI) determined among highly exposed HCWs and low-exposed medical students in Johannesburg, South Africa.
METHODS: We performed a cross-sectional study using both TSTs and IGRAs to determine the prevalence rate of LTBI in 79 medical students and 120 HCWs providing HIV and/or TB care.
RESULTS: The prevalence of LTBI among HCWs was 2- to 4-fold higher than that among medical students (56.7% v. 26.6% TST-positive; 69.2% v.15.2% IGRA-positive, respectively), with 3-fold higher odds for TST positivity and 12-fold higher odds for IGRA positivity among HCWs compared with students. Despite the perception of being at high risk, few HCWs protected themselves against LTBI. The majority of HCWs reported that they would participate in annual TST or IGRA screening.
CONCLUSION: Infection control strategies and occupational screening programmes for professional and lay HCWs, as well as medical students, should be implemented in all high-burden settings. Further research is needed to determine whether IGRA or TST is the optimal assay for periodical screening of HCWs in high-burden settings.
In the 1950s, epidemiological studies in the USA and Europe established tuberculosis (TB) as an occupational hazard, with a increased risk of infection among healthcare workers (HCWs) and paramedical staff compared with the general population.[1] In contrast, occupational TB in developing countries remains a poorly documented problem,[2] but the emergence of extensively drug-resistant TB in HCWs[3] has renewed interest in protecting HCWs in high-burden settings.
The prevalence of latent tuberculosis infection (LTBI) has traditionally been estimated using the tuberculin skin test (TST), with estimates among HCWs in low- and middle-income countries ranging from 33% to 79%.[4] The TST may overestimate the prevalence of LTBI as it detects lifetime cumulative occupational and non-occupational exposure to Mycobacterium tuberculosis, as well as the effects of exposure to non-tuberculous mycobacterial and Bacillus Calmette-Guérin (BCG) vaccination, especially if BCG is administered more than once or after infancy.[4] Furthermore, TST suffers from interobserver variation and a boosting phenomenon if used repeatedly. The interferon gamma release assays (IGRAs), which measure interferon gamma production in whole blood in response to the M. tuberculosis-specific antigenic targets ESAT-6 (6kDa early secreted antigenic target) and CFP-10 (culture filtrate protein-10), may provide more valid estimates of LTBI in HCWs.[5] In 2010, the US Centers for Disease Control and Prevention recommended that both IGRA and TST can be used for periodic screening of HCWs.[6]
Objectives
To (i) assess the prevalence of LTBI among medical students and other highly exposed HCWs in South Africa (SA) using IGRA and TST; (ii) determine factors associated with LTBI; and (iii) assess the risk perception of LTBI in these individuals.
Methods
Setting and participants
This cross-sectional study was part of a larger study of HCWs in Johannesburg, SA. Medical students starting their first year of full-time clinical training were selected because of their low risk of past occupational exposure to M. tuberculosis. Nurses, doctors and counsellors caring daily for patients with active TB or people living with HIV were selected because of their high risk of frequent exposure to M. tuberculosis. Individuals were not eligible for participation if they reported cough for ≥2 weeks, were receiving treatment for active TB at the time of enrolment, or refused HIV testing. All participants provided written informed consent. The study was approved by the ethics review committees of the University of the Witwatersrand and the University of North Carolina at Chapel Hill.
Data collection and measures
Sociodemographic information was collected using a self-administered questionnaire. A non-occupational contact was defined as a friend or family member who had been diagnosed with TB. An LTBI knowledge score (0 - 14) was calculated based on responses to questions on transmission of M. tuberculosis and prevention thereof, diagnosis and course of LTBI, isoniazid preventive therapy, and the effects of HIV and BCG vaccination on TST. Participants were also asked how they viewed their risk of past and future LTBI, their risk of active TB, and their willingness to participate in routine TST or IGRA-based occupational LTBI screening programmes.
HIV testing was performed using an enzyme-linked immunosorbent assay (ELISA) following individual pre-test counselling. Post-test counselling was performed at the next study visit, 24 - 72 hours later. Individuals diagnosed with HIV were referred for care.
The QuantiFERON-TB Gold In-tube (QFT-GIT) IGRA assay (Celestis, Australia) was performed according to manufacturer's instructions at a research laboratory based at the University of the Witwatersrand. A positive QFT-GIT test result was defined as a value ≥0.35 IU/ml for the corrected TB antigen-stimulated plasma level as calculated by the manufacturer's software.
A TST was conducted by injecting two tuberculin units of purified protein derivative (PPD) RT23 intradermally on the volar side of the forearm after blood was drawn for the QFT-GIT and HIV ELISA. The TST was read by one of two investigators 48 - 72 hours after placement and considered positive if induration was ≥10 mm in HIV-negative individuals or ≥5 mm in HIV-positive individuals.
Data analysis
Pearson's χ2 test and Student t-test were used to compare proportions and means between groups. Agreement between TST and IGRA was quantified using the κ statistic. Unadjusted odds ratios (OR), adjusted odds ratios (aOR) and their 95% confidence intervals (CIs) for risk factors associated with LTBI were calculated by multivariate logistic regression using separate models for IGRA or TST outcomes and separate models for medical students and HCWs. Nurses and doctors were collapsed into a single category of professional HCWs. Variables with p-values <0.20 in bivariate analysis were included in the initial multivariate model and removed by a backwards elimination process.
Results
Characteristics of the study population, HIV and LTBI prevalence
In total, 79 medical students and 120 HCWs were enrolled. Of HCWs, 73.3% were nurses. Compared with medical students, HCWs were more likely to be female and older (both p<0.0001) (Table 1).
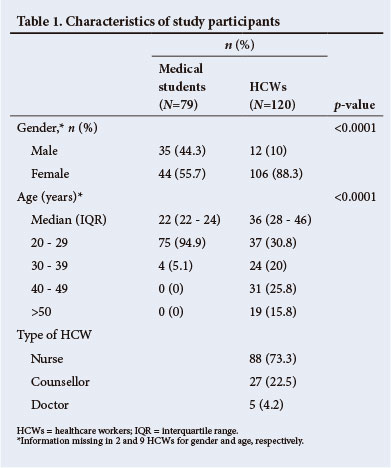
None of the medical students and 18.3% (22/120) of HCWs were HIV-positive (Table 2). A greater proportion of counsellors were HIV-positive than professional HCWs (44.4% v. 10.8%, respectively; p<0.0001). Almost half (48.2%) of all participants reported a non-occupational TB contact, and HCWs were more likely than medical students to report such contact (52.2% v. 41.8%, respectively; p=0.03). HCWs had a lower median LTBI knowledge score than medical students (7 v. 9, respectively; p<0.0001).
Overall, 44.7% (89/199) of participants had a positive TST and 47.7% (95/199) had a positive IGRA. The prevalence of LTBI as measured by TST was higher among HCWs than medical students (56.7% v. 26.6%, respectively; p<0.0001). The distribution of TST induration for medical students and HCWs is shown in Fig. 1. HCWs were more likely than medical students to have a positive IGRA result (69.2% v. 15.2%, respectively; p<0.0001). The prevalence of LTBI was similar in professional HCWs and counsellors. More medical students were positive on TST than on IGRA (26.6% v. 15.2%, respectively; p=0.08), whereas more HCWs were positive on IGRA than on TST (69.2% v. 56.7%, respectively; p=0.045). When comparing the medical students with low exposure to the highly exposed HCWs, the association between intensity of occupational exposure and test positivity was stronger for IGRA (OR 12.25; 95% CI 5.88 - 25.51) than TST (OR 3.56; 95% CI 1.91 - 6.60). The agreement between TST and IGRA was fair (68.4%, κ=0.37).

Factors associated with TST and IGRA positivity
Among medical students, older age (≥24 years) tended to be associated with a positive TST (aOR 2.34; 95% CI 0.78 - 6.97) and positive IGRA (aOR 1.77; 95% CI 0.46 - 6.80), but these differences did not reach statistical significance (Table 3). Report of a non-occupational TB contact was also not associated with a positive TST or IGRA (OR 1.16; 95% CI 0.42 - 3.2 and OR 1.06; 95% CI 0.30 - 3.71). Having a TB knowledge score >7, the median score for all participants, reduced the odds of a positive TST or IGRA by >70%. In multivariate analysis, this association remained statistically significant for TST (aOR 0.29; 95% CI 0.09 - 0.98) but not for IGRA (aOR 0.21; 95% CI 0.07 - 1.16).
Among HCWs, the only association in unadjusted analysis was between HIV status and TST result, with HIV-uninfected HCWs being less likely to have a positive TST (OR 0.28; 95% CI 0.10 - 0.74), but this association was no longer significant after adjustment for age, job category and TB knowledge score (Table 4). Among HCWs, LTBI knowledge score was not associated with a positive result for TST and IGRA.
Perception of LTBI and TB risk and acceptability of occupational screening programmes
Among those who completed the risk assessment questionnaire (83% of students and 30% of HCWs), 27.5% of medical students and 65.7% of HCWs believed they were likely or highly likely to have LTBI, and the majority of students (75.4%) and HCWs (70%) believed it was likely or highly likely that they would acquire LTBI in the next 5 years. Few medical students (16.1%) but many HCWs (52.9%) perceived their risk of developing active TB as likely or highly likely, and 30% of HCWs even believed it was likely they could die of TB. Despite this high-perceived risk, most HCWs (85/111 or 76.6%) reported that they did not take any measures to protect themselves against TB.
Acceptance of a potential, future, free annual occupational screening programme was high, with 90% of medical students and 88% of HCWs reporting they would participate in a TST screening programme, and 96% of medical students and 92% of nurses reporting they would participate in an IGRA screening programme.
Discussion
In Johannesburg, SA, the prevalence of LTBI among HCWs providing care for people with TB and/or HIV was 2- to 4-fold higher than that among medical students finishing their theoretical studies (56.7% v. 26.6% TST-positive; 69.2% v. 15.2% IGRA-positive, respectively), with 3-fold higher odds for TST positivity and 12-fold higher odds for IGRA positivity among highly exposed HCWs compared with less exposed medical students. Except for better knowledge of LTBI in medical students, participant characteristics such as age, non-occupational contact, job category, or HIV status were not associated with LTBI (defined as either positive IGRA or TST) among medical students or HCWs.
The 56.7% TST positivity rate among HCWs falls within the reported 33 - 79% range for low- and middle-income countries.[4] Even though >50 studies have assessed IGRA in HCWs, only 3 studies took place in high TB-burden settings[7-9] and none in sub-Saharan Africa (SSA). The 69.2% positivity rate we observed among SA HCWs was higher than the 40 - 47% positivity found in Indian, Russian and Vietnamese HCWs.[7-9]
The 26.6% TST prevalence among SA medical students recruited at the end of their theoretical training was similar to the 27.1% TST prevalence observed in Zimbabwean nursing students recruited at the start of their training.[10] No data have been published on the prevalence of positive IGRA results among medical or nursing students in SSA. The 15.2% IGRA positivity prevalence rate among medical students was, however, low compared with the 65 - 78% IGRA positivity rate among household contacts of a TB case in SA communities,[11] especially given the report of non-occupational TB contact in 42% of the medical students in our study. This could be due to a longer timespan between contact with a TB case and IGRA in the medical students, higher HIV prevalence rate among household contacts, and higher socioeconomic status of medical students.
More HCWs were IGRA-positive than TST-positive, whereas more medical students were TST-positive than IGRA-positive. IGRA-negative/TST-positive discordances have been observed in all studies of HCWs in low-burden settings,[12] and IGRA positivity was also slightly lower in the only two published studies of LTBI in HCWs in high-burden settings,[7,8] with the difference being significant only in one study.[7] High rates of IGRA-positive/TST-negative discordances have not yet been reported in HCWs but were observed in adult and childhood contacts of new TB cases.[11,13] Similar to the study in India,[8] which found a stronger, albeit non-significant, association between occupational risk and IGRA v. TST positivity, we observed a significantly stronger association between level of occupational risk and IGRA positivity than occupational risk and TST positivity (OR 12.25 for IGRA v. 3.6 for TST). These observations support the hypothesis that IGRA may detect more recent infection (involving CD4+ T-cells of the effector memory phenotype that have recently encountered antigens in vivo), whereas TST detects cumulative exposure to M. tuberculosis over a person's lifetime (involving CD4+ T-cells of the memory phenotype).[14]
Given the repeated nature of occupational screening programmes, test performance is an important determinant, but perceived risk of LTBI and acceptability of the test may also determine the success of such programme. We found that most HCWs perceive themselves to be at high risk of LTBI and active TB, yet few take measures to protect themselves. Almost all, reassuringly, indicated their willingness to participate in an annual occupational TST or IGRA-based screening programme.
While our study is important, as it is the first study to assess both IGRA and TST in medical students and highly exposed HCWs in SSA, there were several limitations shared with other cross-sectional studies of LTBI in HCWs: (i) distinguishing occupational from non-work-related infection was not possible; (ii) owing to the lack of a gold standard for diagnosing LTBI, the cause of discrepant results between IGRA and TST cannot be assessed; (iii) due to the cross-sectional nature of the study, it was not possible to determine the predictive value of a positive IGRA or TST result in predicting progression to active disease.
Conclusion and policy implications
The high LTBI prevalence rates among HCWs, in addition to the 11% HIV prevalence rate among nursing staff, highlights the urgent need to prioritise implementation of infection control strategies[15] and establishment of occupational health programmes in high TB-burden settings.[16] The high rates of TB and HIV co-infection observed among HIV counsellors underscore the need for inclusion of lay workers in occupational screening programmes. The relatively low prevalence rate of LTBI in medical students at the end of their theoretical training exposes their risk of M. tuberculosis acquisition during practice, and underscores the need for screening programmes similar to those in medical schools in high-resource settings.
In 2010, based on limited data and in the absence of any data from SSA, a World Health Organization Expert Committee strongly recommended that IGRAs should not be used in HCW screening programmes in high-burden settings.[17] Our findings of a stronger association between IGRA and occupational risk compared with TST suggest that the choice of assay for occupational screening programmes in high-burden settings requires further study.
Acknowledgements. We thank P Keller and M Tellie for their contributions. We are grateful for the funding received from the North Carolina Occupational Safety and Health Education Research Center (grant no. T 42 OH 008673) and the United States Agency for International Development (USAID)/the US President's Emergency Plan for AIDS Relief (PEPFAR). We thank all participants for their time and dedication to this study.
References
1. Sepkowitz KA. Tuberculosis and the health care worker: A historical perspective. Ann Intern Med 1994;120(1):71-79. [http://dx.doi.org/10.7326/0003-4819-120-1-199401010-00012] [ Links ]
2. Fennelly KP, Iseman MD. Health care workers and tuberculosis: The battle of a century. Int J Tuberc Lung Dis 1999;3(5):363-364. [ Links ]
3. Gandhi NR, Moll A, Sturm AW, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet 2006;368(9547):1575-1580. [http://dx.doi.org/10.1016/S0140-6736(06)69573-1] [ Links ]
4. Joshi R, Reingold AL, Menzies D, Pai M. Tuberculosis among health-care workers in low- and middle-income countries: A systematic review. PLoS Med 2006;3(12):e494. [http://dx.doi.org/10.1371/journalpmed.0030494] [ Links ]
5. Swindells JE, Aliyu SH, Enoch DA, Abubakar I. Role of interferon-gamma release assays in healthcare workers. J Hosp Infect 2009;73(2):101-108. [http://dx.doi.org/10.1016/j.jhin.2009.05.005] [ Links ]
6. Mazurek M, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K; IGRA Expert Committee; Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep 2010;59(RR-5):1-25. [ Links ]
7. Lien LT, Hang NT, Kobayashi N, et al. Prevalence and risk factors for tuberculosis infection among hospital workers in Hanoi, Viet Nam. PLoS One 2009;4(8):e6798. [http://dx.doi.org/10.1371/journal.pone.0006798] [ Links ]
8. Pai M, Gokhale K, Joshi R, et al. Mycobacterium tuberculosis infection in health care workers in rural India: Comparison of a whole-blood interferon gamma assay with tuberculin skin testing. JAMA 2005;293(22):2746-2755. [http://dx.doi.org/10.1001/jama.293.22.2746] [ Links ]
9. Drobniewski F, Balabanova Y, Zakamova E, Nikolayevskyy V, Fedorin I. Rates of latent tuberculosis in health care staff in Russia. PLoS Med 2007;4(2):e55. [http://dx.doi.org/10.1371/journal.pmed.0040055] [ Links ]
10. Corbett EL, Muzangwa J, Chaka K, et al. Nursing and community rates of Mycobacterium tuberculosis infection among students in Harare, Zimbabwe. Clin Infect Dis 2007;44(3):317-323. [http://dx.doi.org/10.1086/509926] [ Links ]
11. Shanaube K, Hargreaves J, Fielding K, et al. Risk factors associated with positive QuantiFERON-TB Gold In-Tube and tuberculin skin tests results in Zambia and South Africa. PLoS One 2011;6(4):e18206. [http://dx.doi.org/10.1371/journal.pone.0018206] [ Links ]
12. Zwerling A, van den Hof S, Scholten J, Cobelens F, Menzies D, Pai M. Interferon-gamma release assays for tuberculosis screening of healthcare workers: A systematic review. Thorax 2012;67(1):62-70. [http://dx.doi.org/10.1136/thx.2010.143180] [ Links ]
13. Adetifa IM, Ota MO, Jeffries DJ, et al. Commercial interferon gamma release assays compared to the tuberculin skin test for diagnosis of latent Mycobacterium tuberculosis infection in childhood contacts in the Gambia. Pediatr Infect Dis J 2010;29(5):439-443. [http://dx.doi.org/10.1097/INF.0b013e3181cb45da] [ Links ]
14. Mack U, Migliori GB, Sester M, et al. LTBI: Latent tuberculosis infection or lasting immune responses to M. tuberculosis? A TBNET consensus statement. Eur Respir J 2009;33(5):956-973. [http://dx.doi.org/10.1183/09031936.00120908] [ Links ]
15. World Health Organization. WHO Policy on TB Infection Control in Health-Care Facilities, Congregate Settings and Households. Geneva: WHO, 2009. http://www.who.int/tb/publications/2009/infection_control/en/index.html (accessed 23 August 2013). [ Links ]
16. World Health Organization. The Joint WHO ILO UNAIDS Policy Guidance on Improving Health Workers' Access to HIV and TB Prevention, Treatment, Care and Support Services. Geneva, WHO: 2010. http://www.who.int/occupational_health/publications/hiv_tb_guidelines/en/ (accessed 23 August 2013). [ Links ]
17. World Health Organization. Use of Interferon Gammma Release Assays (IGRAs) in Tuberculosis Control in Low and Middle-Income Settings. Geneva, WHO: 2011. http://whqlibdoc.who.int/hq/2011/WHO_HTM_TB_2011.17_eng.pdf (accessed 23 August 2013). [ Links ]
 Correspondence:
Correspondence:
W D F Venter
(fventer@wrhi.ac.za)
Accepted 7 June 2013














