Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.103 no.10 Pretoria Jan. 2013
TREATMENT AND CHEMOPROPHYLAXIS
Case management of malaria: Treatment and chemoprophylaxis
I S UkpeI, II; D MoonasarIII; J RamanIV; K I BarnesV; L BakerVI; L BlumbergVII
IDepartment of Family Medicine, University of Pretoria, South Africa
IIMpumalanga Department of Health, Nelspruit, South Africa
IIIMalaria Directorate, National Department of Health, Pretoria, South Africa
IVMalaria Research Unit, South African Medical Research Council, Durban, South Africa
VDivision of Clinical Pharmacology, University of Cape Town, South Africa
VIAmayeza Drug Information Centre, Johannesburg, South Africa
VIIDivision of Public Health Surveillance and Outbreak Response, National Institute for Communicable Diseases, Johannesburg, South Africa
ABSTRACT
Malaria case management is a vital component of programmatic strategies for malaria control and elimination. Malaria case management encompasses prompt and effective treatment to minimise morbidity and mortality, reduce transmission and prevent the emergence and spread of antimalarial drug resistance. Malaria is an acute illness that may progress rapidly to severe disease and death, especially in non-immune populations, if not diagnosed early and promptly treated with effective drugs. In this article, the focus is on malaria case management, addressing treatment, monitoring for parasite drug resistance, and the impact of drug resistance on treatment policies; it concludes with chemoprophylaxis and treatment strategies for malaria elimination in South Africa.
Malaria case management is a vital component of programmatic strategies for malaria control and elimination.[1-3] Malaria case management encompasses prompt and effective treatment to minimise morbidity and mortality, reduce transmission and prevent the emergence and spread of antimalarial drug resistance.[1-3] The number of malaria-related deaths is a key epidemiological indicator in malaria programmes used to evaluate performance in delivering effective malaria case management.[1]
Malaria is an acute illness that may progress rapidly to severe disease and death, especially in non-immune populations, if not diagnosed early and promptly treated with effective drugs. South Africans, including those living in the malaria transmission areas within the country, are generally non-immune. All age groups are therefore at risk of developing severe disease when infected.[4,5]
Some important considerations to ensure effective malaria case management include availability and accessibility of antimalarial medicines, training of healthcare workers at all levels of healthcare delivery and dealing with the problem of antimalarial drug resistance.[1] In this article, the focus is on malaria case management, addressing treatment, monitoring for parasite drug resistance, and the impact of drug resistance on treatment policies; it concludes with chemoprophylaxis and treatment strategies for malaria elimination in the country.
1. Malaria treatment in endemic and non-endemic provinces
Stratification of malaria risk areas in South Africa (SA) into endemic and non-endemic areas followed the first malaria survey in the country in 1921 by Park Ross, who was then the Assistant Secretary of Health of the Union of SA. Malaria-endemic parts at that time included the Pretoria and Durban districts.[6,7] Malaria endemic areas in today's SA are the north-eastern part of KwaZulu-Natal (KZN) Province and the low altitude regions of Mpumalanga and Limpopo provinces.[4,8] Although the Northern Cape and North West provinces are classified as non-endemic, malaria transmission occurs occasionally in areas adjacent to the Molopo and Orange rivers.[5] Malaria treatment strategies in the country differ slightly in endemic and non-endemic provinces.
Historically, before the advent of chloroquine (CQ) in the late 1940s, quinine was used for both treatment and prophylaxis, but the measures could not be practically extended to the population in the endemic areas as a whole.[6,7] During the 1970s, the malaria control programme in the country became more structured. Treatment was based on definitive diagnosis by blood smear microscopy. Malaria case detection and treatment was mainly through active case finding during house-to-house surveys and mass blood examination and between 1987 and 1990, >50% of all malaria cases in the country were detected by active case finding. [6,7] Passive case detection and treatment, whereby symptomatic patients at healthcare facilities were tested for malaria and treated if found positive only played a minor role in parasite control. In KZN Province, 30% of all cases in the same period were detected at hospitals and clinics, of which clinics contributed only 12%.[6,7]
In the 1990s, Sharp et al.[6,7] hypothesised that the relatively minor role of clinics in malaria case detection in the country could be addressed in a cost-effective manner by community involvement and enhanced service at the clinic level, effectively reducing the need for active case detection, and reducing costs. This was realised in the mid-1990s. The adoption of the District Health System (DHS) based on primary healthcare (PHC) as the healthcare strategy for SA since 1994,[9] the subsequent integration of passive diagnosis and treatment of malaria into PHC within the DHS[10] and the introduction of malaria rapid diagnostic tests (RDTs) at the PHC level starting in Mpumalanga Province in 1996,[11] revolutionised malaria case detection and treatment across the country. Passive detection and treatment of malaria at healthcare facilities based on a parasitological diagnosis with RDTs at PHC facilities and prompt, effective treatment is currently the major malaria case management strategy to reduce transmission in endemic areas. In Mpumalanga Province, passive case detection contributes >90% of reported total malaria cases each year in this era of PHC-based DHS in SA (Fig. 1) (Mpumalanga Malaria Programme, unpublished data).

Malaria diagnosis and treatment are provided free-of-charge at public healthcare facilities in both endemic and non-endemic provinces in the country. Evidence-based national or provincial guidelines informed by expert recommendations dictate the selection of preferred antimalarials, and drug choice policies have differed between provinces at various times (Table 1). Drug resistance surveillance data driving these policies are discussed later.

Currently in all endemic and non-endemic provinces, artemether-lumefantrine (AL) is the recommended first-line antimalarial medicine for the treatment of uncomplicated falciparum malaria. Primaquine is used for radical cure of Plasmodium vivax and P. ovale infections.[5] Primaquine is currently unregistered but is available through a Section 21 process.[5] The second-line antimalarial medicines for the treatment of uncomplicated malaria remain oral quinine plus doxycycline (for adults) or clindamycin (for pregnant women and children under the age of 8 years).[5]
Parenteral quinine has been the mainstay of treatment of severe malaria, and is still widely used in most African countries today. [12,13] Intravenous artesunate is a new parenteral antimalarial currently recommended by the World Health Organization (WHO) for the treatment of severe malaria in adults and children.[2,14] Intravenous artesunate is not yet registered for use in SA, but there is limited availability through a special access programme for compassionate use on a named-patient basis.[5]
In the endemic provinces, treatment with the recommended first-line antimalarial medicines for uncomplicated malaria is accessible at any level of public healthcare facility, including fixed and mobile PHC facilities. Parenteral antimalarial medicines for the treatment of severe malaria are only available at hospital level. In the non-endemic provinces, antimalarial treatments are mostly accessible at the hospital level. In both endemic and non-endemic provinces, uncomplicated malaria is treated at outpatient level, except for high-risk population groups (pregnant and postpartum women, infants and young children, the elderly (>65 years) and immunocompromised patients, including those with HIV/AIDS), who constitute a definite indication for hospital admission.[5] Severe malaria is a medical emergency that requires high-level hospital care; thus, for severe malaria patients diagnosed at PHC and private general practitioner facilities, emergency transfer to hospital is the norm in both endemic and non-endemic provinces.[5] The WHO recommends pre-referral treatment of severe malaria with intramuscular artesunate, artemether, quinine or rectal artesunate if the transfer time to hospital is longer than 6 hours.[2] This recommendation is not implemented in SA.
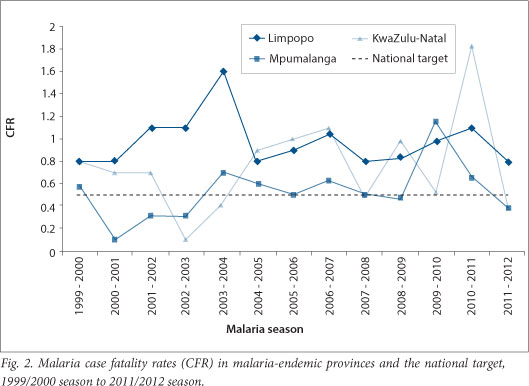
The major challenge facing malaria case management in the country is reduction of malaria-related deaths. Late presentation, lack of awareness of malaria in communities, and a low index of suspicion in healthcare workers, particularly in non-endemic provinces in the country,[15-18] are major contributing factors to malaria-related deaths. Even in the endemic provinces, case fatality rates (CFRs), defined as the number of deaths per 100 cases of malaria is above the national target of <0.5% (Fig. 2). The importance of continuing education of healthcare workers on malaria diagnosis and treatment[19] is critical. Regular training of healthcare workers at PHC and hospital levels on the diagnosis and management of uncomplicated and severe malaria is a major malaria case management intervention activity in both endemic and non-endemic provinces in the country.
2. Antimalarial drug resistance across SA and neighbouring countries
Antimalarial drug resistance has greatly influenced malaria case management strategies in SA since the 1'80s. Stringent control interventions comprising indoor residual spraying, periodic larviciding, and treatment with CQ from the late 1940s ensured malaria posed no severe public health burden until the mid-1980s.[20] Unfortunately by this time CQ resistance had reached Africa and was becoming firmly entrenched in southern Africa.[21]
2.1 KZN
KZN Province first reported in vitro CQ resistance in 1985.[22] Under sustained CQ pressure, resistance spread, resulting in an increase in malaria cases and treatment failures. By 1987, 3% of all malaria-treated patients remained malaria-positive despite being treated four times with CQ.[23] The rise in cases and treatment failures prompted the KZN Department of Health to replace CQ with sulphadoxine-pyrimethamine (SP) in 1988. This policy change caused case numbers to gradually decline from a high of 6 757 in 1987 to <500 by 1992.
However, between 1993 and 2000 malaria case numbers began rising sharply, peaking in 2000 with over 40 000 cases reported in KZN.[24] An in vivo efficacy trial revealed the 42-day cure rate following SP treatment had fallen from above 75% in 1997 to 11% by 2000.[25] Retrospective genetic analyses on samples collected during a community-based prevalence survey in 2000 confirmed the presence of highly SP-resistant parasites, with 47% of all parasites analysed in carrying the SP quintuple mutation associated with SP treatment failure.[26] This high prevalence of SP-resistant parasites most likely enhanced transmission, as individuals infected with SP-resistant parasites have increased gametocyte loads,[27] which are more infectious to mosquitoes than SP-sensitive gametocytes.
KZN responded to this SP resistance epidemic by becoming the first province to deploy the artemisinin-containing combination treatment (ACT), AL, in 2001.[24] Although malaria morbidity declined by 99% following this policy change,[24] the SP quintuple mutation remained extremely prevalent in the province. By 2012, 75% of the parasites analysed carried the mutation (J Raman, unpublished data). Sustained antimicrobial pressure by co-trimoxazole, an antifolate-sulphonamide combination used as prophylaxis against opportunistic infections in HIV/AIDS patients[28] may be the reason, given the high HIV/AIDS prevalence in KZN.
Encouragingly, however, parasites carrying molecular markers associated with CQ sensitivity, namely, crtK76T and mdr186N[29] are beginning to resaturate the KZN P falciparum population.[30] A similar phenomenon has been seen in both Mozambique[31] and Malawi[32] following the prolonged removal of CQ drug pressure. This re-emergence of CQ sensitivity may allow for the use of CQ as an antimalarial partner drug in the future.
2.2 Mpumalanga and Limpopo
Like KZN Province, reports of CQ resistance from Mpumalanga[23] and Limpopo provinces[33] first emerged in the 1980s. However, CQ treatment failures in both of these provinces remained relatively rare until the 1990s. A possible reason for the delay in treatment failures is the lower incidence of immigrant asymptomatic malaria carriers entering Mpumalanga and Limpopo provinces compared with KZN. The border between KZN and Mozambique is extremely porous, with people crossing the border on a daily basis.[6] A border-screening malaria survey in 1996 revealed 58% of the individuals entering KZN were asymptomatic malaria carriers who remained untreated in the province for long periods, thereby contributing to local transmission.[20]
Both in vitro and in vivo studies revealed a growing population of CQ-resistant parasites in both Mpumalanga and Limpopo provinces[21,34] in the 1990s, which led to SP becoming the antimalarial of choice in Mpumalanga Province in 1997 and a year later in Limpopo Province. Although therapeutic efficacy studies demonstrated sustained SP efficacy in Mpumalanga five years after SP introduction,[35] with SP quintuple mutation prevalence only at 10% (J Raman, unpublished data), patients successfully treated with SP displayed enhanced gametocyte carriage.[35]
In an attempt to reduce gametocyte carriage and malaria case numbers the ACT, artesunate-SP, replaced SP as the antimalarial of choice in Mpumalanga Province in 2001. While this change resulted in the quintuple mutation prevalence declining to <10%, malaria case numbers remained largely unchanged in the next few years. The low SP quintuple mutation prevalence suggested that something other than SP resistance was sustaining malaria transmission.
In accordance with the South African National Malaria Treatment guidelines, AL became the first-line treatment in Mpumalanga in 2006. This policy change was very timely as the SP quintuple mutation prevalence had risen to 48% by 2007 (J Raman, unpublished data). As seen in KZN, AL introduction resulted in a decline in malaria cases, but had no effect on quintuple mutation prevalence. By 2011 confirmed malaria case numbers had declined by 35% while quintuple mutation prevalence has risen to above 80% (J Raman, unpublished data). The crt76T and mdr186N molecular markers associated with CQ sensitive had become re-established in the population and were approaching fixation by 2011 (J Raman, unpublished data).
Although AL became the first-line treatment in Limpopo Province in 2004, there was no major decline in malaria case numbers by 2011. Unfortunately, drug resistance surveillance data are limited, with only one study in 2010 showing quintuple mutation at 41% and <1% of parasites analysed carrying markers associated with CQ resistance.
2.3. Regional drug pressure
Despite stringent efforts, drug-resistant malaria epidemics still occurred in SA, in part due to the importation of drug-resistant parasites. Resistance to both CQ and SP arose in Southeast Asia and spread into Africa via gene flow rather than evolving de novo,[26,36] highlighting the need for regional malaria control, and more importantly, a clear understanding of the malaria epidemiology in neighbouring countries. As the movement of both the malaria vector and parasite is not restricted by national boundaries regional drug pressure can influence drug efficacy within countries, as seen in KZN Province, SA[20] and Maputo Province, Mozambique.[37] Given the first-line antimalarial in all southern African countries is AL and that artemisinin resistance has been confirmed in Southeast Asia,[38] routine monitoring of AL efficacy is recommended, particularly in Limpopo Province, which currently experiences the highest incidence of African immigrant visitors.
3. Antimalarial drug efficacy monitoring and the impact of drug resistance on treatment policies
The inevitable emergence and spread of drug resistant parasites has had profound effects on South African malaria case management strategies. Thus, sustained and rigorous monitoring for antimalarial resistance provides the essential backbone for informing evidence-based malaria treatment policies. During the malaria control phase, such monitoring can effectively be conducted through regular in vivo therapeutic efficacy studies. Such studies were conducted regularly in all three malaria endemic provinces (KZN, Mpumalanga and Limpopo) until 2004. These data were complemented by data on antimalarial drug exposure and molecular markers of resistance for the treatments recommended. In vitro assays have occasionally been used to monitor for antimalarial resistance in SA.[21,34]
The dramatic reductions in local malaria transmission following strengthening of vector control and large-scale deployment of ACT subsequently precluded an adequate sample size being recruited in any sentinel site in SA. Drug resistance monitoring is currently based primarily on monitoring for known molecular markers of resistance, complemented by surveillance to define geographic and temporal trends in malaria case numbers, and routine follow-up of malaria cases post-treatment to detect potential treatment failures.
Malaria treatment policies are decentralised to the provincial level, which has resulted in the three malaria endemic provinces at times having different policies, as summarised in Table 1. The major driver for changes in malaria treatment policies in SA has been evidence of parasite resistance to the recommended treatment reaching unacceptable levels, as outlined above. Factors usually considered for the selection of each new treatment policy include recommendations by the WHO, regulatory requirements of the South African Medicines Control Council, international peer-reviewed evidence on efficacy, safety and tolerability, cost, and likelihood of compliance and adherence (including duration and complexity of treatment course).
In KZN, AL was selected as the preferred ACT as there was no established resistance to the partner drug, lumefantrine, unlike SP or amodiaquine. By contrast, in Mpumalanga and Limpopo, where SP remained highly effective, the ACT artesunate-SP was selected. This combination had the advantage of not requiring co-administration with fat and the majority of patients likely to be cured even if they were not fully adherent, given that SP treatment only requires a single dose. However, a number of disadvantages were detected with careful monitoring following the deployment of this combination, including: suboptimal SP exposure in young children given the recommended dose of SP (K Barnes, unpublished data); that it could not be manufactured as a fixed dose combination, and so risked use of artesunate monotherapy; and that molecular markers of SP resistance continued to increase despite SP being used in combination with artesunate (J Raman, unpublished data).
However, additional factors have influenced policy and practice. The malaria control programme in KZN added a single dose of primaquine to SP in the mid-1990s, to reduce transmission. This strategy was curtailed when the manufacturer, Winthrop, withdrew the product from the South African market. Primaquine is currently only available for compassionate use on a named-patient basis.[5] Clinicians in KZN started using CQ in addition to SP in the late 1990s when they suspected clinically that SP efficacy was waning, although no formal resistance studies were conducted.
4. Malaria chemoprophylaxis
Malaria chemoprophylaxis entails the use of antimalarial medicines to prevent malaria especially in non-immune people travelling to malaria endemic areas.[3] In SA, chemoprophylaxis is recommended for residents in non-endemic areas travelling to endemic areas within and outside the country.[39] Up until the early 1990s, CQ was the recommended antimalarial medicine for chemoprophylaxis. It could be given to pregnant women and young children. CQ plus pyrimethamine was also used, but in 1991 it was determined that due to pyrimethamine resistance, little benefit was conferred by the combination. There was an increase in side-effects, and a folic acid supplement was necessary when given to pregnant women.[40] CQ alone was therefore preferable. Dapsone-pyrimethamine was another potential option but had a number of side-effects such as agranulocytosis, methaemoglobinaemia and megaloblastic anaemia. These, however, were more common when the recommended dose was exceeded.[40] There was also concern over efficacy and it was therefore not usually officially recommended.[40]
CQ plus proguanil was another option recommended internationally; however, proguanil was only registered in SA in 1997, at which time the combination became the chemoprophylaxis of choice. It was, however, a complicated regime as CQ was taken once weekly and proguanil daily. Up until this point, all chemoprophylactic options were classified Schedule 1 and could thus be given out without a prescription. They were also available from those who were employed by any department responsible for environmental affairs or tourism at a provincial government level, such as the tourist shops in the Kruger National Park.
The emergence of widespread CQ resistance eventually led to its abandonment as antimalarial medicine for chemoprophylaxis. Thereafter, the selection of antimalarial medicines for chemoprophylaxis recommendations in the country has been following the WHO recommendations for CQ-resistant malaria transmission areas. Currently, doxycycline, mefloquine, and atovaquone-proguanil are the recommended choices.[39,41] Unlike CQ and the other earlier medicines, these drugs are only available on prescription and are not free of charge, even in the public healthcare sector.
5. considerations for malaria elimination
5.1 Drug efficacy monitoring
As countries undergo transition towards malaria elimination, routine monitoring of drug efficacy is essential, particularly as any artemisinin-resistant malaria outbreak could result in severe morbidity and mortality given the low or absent immunity to malaria within the population and current dependence on ACT. Conducting in vivo efficacy trials in regions approaching malaria elimination is virtually impossible due to the low case numbers. Hence, alternative methods such as the routine surveillance of molecular resistance markers should be considered. Molecular analysis should be conducted on samples collected from various sentinel sites to ensure robust data is obtained. Technological advances now mean that a liquid blood sample is no longer needed for molecular analysis. Filter paper blood spots and even malaria-positive RDTs[42] can be used as sources of parasite DNA. This routine surveillance must become part of the elimination agenda in all South African malaria endemic regions, particularly in malaria hot spots.
Inability to monitor for molecular markers of resistance rapidly and effectively during the pre-elimination phase is a major threat to our ability to eliminate malaria. Drug resistance has almost invariably emerged in areas of very low-intensity malaria transmission. As the population in these areas is non-immune, infections are usually symptomatic so most patients will seek treatment, thereby increasing drug pressure. Furthermore, they lack the immunity that is needed to suppress replication of resistant parasites. With artemisinin resistance already confirmed in four countries in the Greater Mekong Region of Southeast Asia, and no molecular marker yet validated for detecting artemisinin-resistant parasites, there is a real possibility of this resistance spreading to (or emerging in) SA.
Because malaria cases have become too few at any single health facility in the endemic provinces in the country, a complete in vivo therapeutic efficacy study is not feasible. Until validated molecular markers are available for artemisinin resistance, our best options are to continue to monitor routinely for molecular markers of resistance to lumefantrine (or future ACT partner drugs), and whenever possible to test for the presence of P. falciparum parasites 3 days post-treatment, and to follow up patients 4 - 6 weeks post-treatment, to establish whether they remain malaria-free. The result of the malaria blood smear on day 3 (72 hours post-treatment) is a good predictor of subsequent treatment failure, and provides a simple screening measure for artemisinin resistance. Artemisinin resistance is highly unlikely if the proportion of patients with parasite densities of <100 000 parasites/ml, who have a positive smear result on day 3 after ACT treatment, is <3%.[43]
5.2 Radical cure
The malaria situation in SA has now moved from control phase to the pre-elimination and elimination phases. ACT policy is fully implemented in the country. As malaria becomes less common, finding and treating the last few cases becomes increasingly onerous and expensive, so it is important to maximise all opportunities for interrupting transmission. One such intervention, which is endorsed by the WHO,[44] is to treat all positive malaria cases with a single dose of primaquine. This drug efficiently inactivates gametocytes but in persons with genetically determined glucose-6-phosphate dehydrogenase (G6PD) enzyme deficiency of red blood cells, it may cause haemolysis and subsequent anaemia of variable severity. Historically, the South African black population has a low rate of G6PD deficiency, and the enzyme defect locally is generally quantitatively fairly mild, so severe reactions are uncommon.[45] However, with increasing immigration from other parts of Africa, the demographics of G6PD deficiency may have changed. Before the elimination programme decides to go ahead with primaquine use, it needs to update local knowledge about G6PD deficiency in SA, for ethical and scientific reasons.
5.3 Community-level treatment
As part of the malaria elimination programme, SA is seriously considering community-level treatment of uncomplicated and asymptomatic malaria detected during active case investigation by malaria surveillance officers. The feasibility of community-level treatment will require extensive training of community health workers and malaria field investigators, as well as drug regulatory changes, for this to happen.
5.4 Free prophylaxis
The currently recommended chemoprophylactic options, which are all very effective if taken correctly, are mefloquine, doxycycline and atovaquone-proguanil.[39,41] They are all only available on prescription. This significantly hinders accessibility by the public. As SA moves towards elimination, it will be imperative to make these products readily accessible to all those travelling to malaria-risk areas, including those who can ill afford chemoprophylaxis. This is important not only for reducing the risk of the travellers becoming sick with malaria but also for elimination purposes - the prevention of importation of parasites into the country which could result in infection of local mosquitoes and local malaria transmission.
While there is a strong case and support for the provision of free chemoprophylaxis for travellers to malaria-endemic areas for malaria elimination purposes, questions around feasibility, cost effectiveness, regulation, accessibility and drug scheduling will need to be answered before the strategy can be considered in SA.
References
1. World Health Organization. Malaria Case Management Operations Manual. Geneva: WHO, 2009. http://www.who.int/iris/handle/10665/44124 (accessed 12 August 2013). [ Links ]
2. World Health Organization. Guidelines for the Treatment of Malaria - 2nd Edition. Geneva: WHO, 2010. http://www.who.int/iris/handle/10665/44227 (accessed 12 August 2013). [ Links ]
3. World Health Organization. Malaria Elimination: A Field Manual for Low and Moderate Endemic Countries. Geneva: WHO, 2007. http://www.who.int/iris/handle/10665/43796 (accessed 12 August 2013). [ Links ]
4. Blumberg L, Frean J. Malaria control in South Africa - challenges and successes. S Afr Med J 2007;97(11):1193-1197. [ Links ]
5. South African National Department of Health. Guidelines for the Treatment of Malaria in South Africa. Pretoria: NDoH, 2010. [ Links ]
6. Sharp BL, Le Sueur D. Malaria in South Africa - the past, the present and selected implications for the future. S Afr Med J 1996;86:83-89. [ Links ]
7. Sharp B, Craig M, Curtis B et al. Malaria. In: Health Systems Trust. South Africa Health Review 2000. Durban: Health Systems Trust, 2000. http://www.hst.org.za/publications/south-african-health-review-2000 (accessed 30 July 2013). [ Links ]
8. Moonasar D, Nuthulaganti T, Kruger PS, et al. Malaria control in South Africa 2000-2010: Beyond MDG6. Malar J 2012;11:294. [http://dx.doi.org/10.1186/1475-2875-11-294] [ Links ]
9. Health Systems Trust. Bringing Health Closer to People: Local Government and the District Health System. Durban: Health Systems Trust, 2001. http://www.hst.org.za/all-publications (accessed 30 July 2013). [ Links ]
10. South African National Department of Health. White Paper for the Transformation of the Health System in South Africa. Pretoria: NDoH, 1997. http://www.doh.gov.za/index.php (accessed 30 July 2013). [ Links ]
11. Durrheim DN, la Grange JJ, Govere J, et al. Accuracy of a rapid immunochromatographic card test for Plasmodium falciparum in a malaria control programme in South Africa. Trans R Soc Trop Med Hyg 1998;92:32-33. [ Links ]
12. Achan J, Talisuna AO, Erhat A, et al. Quinine, an old anti-malarial drug in a modern world: Role in the treatment of malaria. Malar J 2011;10:144. [http://dx.doi.org/10.1186/1475-2875-10-144] [ Links ]
13. World Health Organization. Global Antimalarial Drug Policies Database - WHO African Region December 2012 Update. Geneva: WHO, 2012. [ Links ]
14. World Health Organization. Guidelines for the Treatment of Malaria - 2nd Edition, Rev. 1. Geneva: WHO, 2011. [ Links ]
15. Spencer-Jones JH. Malaria update. S Afr Med J 1996;86:622-624. [ Links ]
16. Soni PN, Gouws E. Severe and complicated malaria in KwaZulu-Natal. S Afr Med J 1996; 86:653-656. [ Links ]
17. Durrheim DN, Frieremans S, Kruger P, et al. Confidential inquiry into malaria deaths. Bull World Health Organ 1999;77:263-266. [ Links ]
18. Mehta U, Durrheim DN, Blumberg L, et al. Malaria deaths as sentinel events to monitor healthcare delivery and antimalarial drug safety. Trop Med Int Health 2007;12:617-628. [http://dx.doi.org/10.1111/j.1365-3156.2007.01823.x] [ Links ]
19. Ukpe IS. Continuing medical education in unstable malaria areas. Bull World Health Organ 1999;77:948. [ Links ]
20. Craig MH, Kleinschmidt I, Le Sueur D, et al. Exploring 30 years of malaria case data in KwaZulu-Natal, South Africa: Part II. The impact of non-climatic factors. Trop Med Int Health 2004;9(12):1258-1266. [http://dx.doi.org/10.1111/j.1365-3156.2004.01341.x] [ Links ]
21. Deacon HE, Freese JA, Sharp BL. Drug resistant Plasmodium falciparum malaria in the eastern Transvaal. S Afr Med J 1994;84:394-395. [ Links ]
22. Herbst JM, Taylor LA, Joubert SM. Chloroquine resistance in Plasmodium falciparum in Natal. S Afr Med J 1987;72:627-629. [ Links ]
23. Hansford CF. Chloroquine resistance in Plasmodium falciparum in KwaZulu, 1983 - 1988. S Afr Med J 1989;76:546-547. [ Links ]
24. Barnes KI, Durrheim DN, Little F, et al. Effect of artemether-lumefantrine policy and improved vector control on malaria burden in KwaZulu-Natal, South Africa. PLoS Med 2005;2:e330. [http://dx.doi.org/10.1371/journal.pmed.0020330] [ Links ]
25. Freese JA, Sharp BL, Ngxongo SM, et al. In vitro confirmation of chloroquine-resistant Plasmodium falciparum malaria in KwaZulu. S Afr Med J 1988;74:576-578. [ Links ]
26. Roper C, Pearce R, Bredenkamp B, et al. Antifolate antimalarial resistance in southeast Africa: A population-based analysis. Lancet 2003;361(9364):1174-1181. [http://dx.doi.org/10.1016/S0140-6736(03)12951-0] [ Links ]
27. Barnes KI, Little F, Mabuza A, et al. Increased gametocytemia after treatment: An early parasitological indicator of emerging sulfadoxine-pyrimethamine resistance in falciparum malaria. J Infect Dis 2008;197:1605-1613. [http://dx.doi.org/10.1086/587645] [ Links ]
28. White NJ. Antimalarial drug resistance. J Clin Invest 2004;113:1084-1092. [http://dx.doi.org/10.1172/JCI200421682] [ Links ]
29. Djimde A, Doumbo OK, Cortese JF, et al. A molecular marker for chloroquine-resistant falciparum malaria. N Engl J Med 2001;344:257-263. [http://dx.doi.org/10.1056/NEJM200101253440403] [ Links ]
30. Vaughan-Williams CH, Raman J, Raswiswi E, et al Assessment of the therapeutic efficacy of artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparium malaria in northern KwaZulu-Natal: An observational cohort study. Malar J 2012;11:434. [http://dx.doi.org/10.1186/1475-2875-11-434] [ Links ]
31. Raman J, Mauff K, Muianga P, et al Five years of antimalarial resistance marker surveillance in Gaza Province, Mozambique, following artemisinin-based combination therapy roll out. PloS One 2011;6:e25992. [http://dx.doi.org/10.1371/journal.pone.0025992] [ Links ]
32. Kublin JG, Cortese JF, Njunju EM, et al. Re-emergence of chloroquine-sensitive Plasmodium falciparum malaria after the cessation of chloroquine use in Malawi. J Infect Dis 2003;187:1870-1875. [ Links ]
33. Visagie NJ, Sieling WL. Chloroquine-resistant Plasmodium falciparum malaria in South Africa. S Afr Med J 1985;68:600-601. [ Links ]
34. Govere JM, la Grange JJ, Durrheim DN, et al Sulfadoxine-pyrimethamine effectiveness against Plasmodium falciparum malaria in Mpumalanga Province, South Africa. Trans R Soc Trop Med Hyg 1999;93:644. [http://dx.doi.org/10.1016/S0035-9203(99)90082-2] [ Links ]
35. Mabuza A, Govere J, la Grange K, et al Therapeutic efficacy of sulfadoxine-pyrimethamine for Plasmodium falciparum malaria. S Afr Med J 2005;95:346-349. [ Links ]
36. Payne D. Spread of chloroquine resistance in Plasmodium falciparum. Parasitol Today 1987;3:241-246. [ Links ]
37. Raman J, Sharp B, Kleinschmidt I, et al. Differential effect of regional drug pressure on dihydrofolate reductase and dihydropteroate synthetase mutations in southern Mozambique. Am J Trop Med Hyg 2008;78: 256-261. [ Links ]
38. Dondorp AM, Nosten F, Poravuth Y, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 2009;361:455-467. [http://dx.doi.org/10.1056/NEJMoa0808859] [ Links ]
39. South African National Department of Health. Guidelines for the Prevention of Malaria in South Africa 2009. Pretoria: NDoH, 2009. [ Links ]
40. Baker L, van Schoor JD, Bartlett GA, et al. Malaria prophylaxis - the South African viewpoint. S Afr Med J 1993;83:126-129. [ Links ]
41. Baker L. Malaria prophylaxis - make the right choice for travellers with special circumstances. South Afr J Epidemiol Infect 2009;24(4):44-49. [ Links ]
42. Ishengoma DS, Lwitiho S, Madebe RA, et al. Using rapid diagnostic tests as source of malaria parasite DNA for molecular analyses in the era of declining malaria prevalence. Malar J 2011;10:6. [http://dx.doi.org/10.1186/1475-2875-10-6] [ Links ]
43. Stepniewska K, Ashley E, Lee SJ, et al. In vivo parasitological measures of artemisinin susceptibility. J Infect Dis 2010;201:570-579. [http://dx.doi.org/10.1086/650301] [ Links ]
44. World Health Organization, WHO Evidence Review Group. The Safety and Effectiveness of Single Dose Primaquine as a P. falciparum Gametocytocide. Geneva: WHO, 2012. http://www.who.int/malaria/mpac/sep2012/primaquine_single_dose_pf_erg_meeting_report_aug2012.pdf (accessed 13 August 2013). [ Links ]
45. Beighton P, Botha MC. Inherited disorders in the black population of southern Africa. Part I. Historical and demographic background; genetic haematological conditions. S Afr Med J 1986;15;69(4):247-249. [ Links ]
 Correspondence:
Correspondence:
D Moonasar
(moonad@health.gov.za)














