Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.102 no.4 Pretoria Abr. 2012
ORIGINAL ARTICLES
Paediatric liver transplantation in Johannesburg: Initial 29 cases and prospects for the future
J A LovelandI; T GovenderII; J BothaIII; R BritzIV
IMB BCh, FCS (SA), Cert Paed Surg. Department of Paediatric Surgery, Chris Hani Baragwanath Academic Hospital; and Division of Transplantation, Wits Donald Gordon Medical Centre and University of the Witwatersrand, Johannesburg
IIMB BCh. Division of Transplantation, Wits Donald Gordon Medical Centre and University of the Witwatersrand
IIIMB ChB, FCS (SA). Division of Transplantation, Wits Donald Gordon Medical Centre and University of the Witwatersrand
IVDA (SA), FCS (SA). Division of Transplantation, Wits Donald Gordon Medical Centre and University of the Witwatersrand
ABSTRACT
BACKGROUND: The Wits Donald Gordon Medical Centre paediatric liver transplant programme is the second such unit in sub-Saharan Africa. Initiated in November 2005, it forms part of the centre's solid organ transplant unit, comprising kidney, liver and simultaneous kidney-pancreas arms. Initially established in the private sector, we recently received government approval to expand our programme into the provincial sector and have performed transplants on several provincial-sector patients. Current challenges relate to the lack of appropriately trained paediatric sub-specialists, specifically critical care practitioners and hepatologists.
METHODS: Subsequent to institutional approval, a retrospective chart analysis of all paediatric liver transplants performed at our facility to date was conducted.
RESULTS: Defining children as those under 18 years of age, 29 patients have received a cadaveric liver transplant since 2005, using 16 whole livers, 10 reduced-size grafts, and 3 split segments; 13 were transplanted with biliary atresia, 3 hyperoxalurea, 3 autosomal recessive polycystic disease, 2 alpha-1 antitrypsin deficiency, and 2 idiopathic, with the remainder for a wide spectrum of other pathologies. Seven patients received combined liver-kidney transplants. There were 3 in-hospital mortalities. The remaining 26 patients are all long-term survivors. We describe 7 acute surgical morbidities in 6 patients, and 8 long-term surgical morbidities. One patient was subsequently re-transplanted in Cape Town.
CONCLUSIONS: Despite a shortage of organs, we have overcome a steep learning curve, with results comparable with other early series. The current threat to the continued viability of our unit is the lack of appropriately trained paediatric hepatologists and intensivists.
Professors Myburgh and Du Plessis established kidney transplantation at the University of the Witwatersrand (Wits) in the 1960s. The unit was successfully run in the provincial and private sectors for many years and was transiently involved in liver transplantation during its inception in the late 1980s. However, the ambition to add liver transplantation to the spectrum of the unit was not supported by national government, who saw no need for a second liver transplant unit in the academic sector in South Africa - the unit at Groote Schuur Hospital in Cape Town was considered sufficient. Fortuitously, Wits' ambition to expand coincided with the establishment of the Wits Donald Gordon Medical Centre (Wits DGMC), a partnership between Wits and the private healthcare sector, which was identified as an ideal environment to launch Johannesburg's liver transplant unit.
Initially catering only for patients with medical insurance, the first adult liver transplant was performed in August 2004. Subsequent to extensive negotiation, both funded and indigent state patients were placed on a common waiting list, and are currently transplanted according to blood group and severity of disease. The unit has developed into a programme offering a near-complete spectrum of transplant solutions, including well-established kidney, liver and simultaneous kidney-pancreas options, to an increasing recipient pool. This has made liver transplantation in northern South Africa more accessible and practical. Our paediatric liver programme started in November 2005, and we have performed 29 transplants to date.
Patients and methods
After receiving institutional approval, we performed a retrospective chart review of all paediatric liver transplants. Paediatric patients are defined as those under 18 years of age on the day of transplantation, and were referred from all areas of South Africa and neighbouring countries. Foreign patients were only considered for transplantation after central government approval.
Results
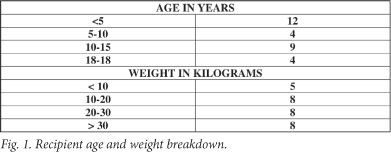
Recipient age and weight breakdown is shown in Fig. 1. Organs were allocated according to appropriate blood grouping, paediatric end-stage liver disease (PELD) score, and size. The United Network for Organ Sharing has used the PELD scoring system as a means of prioritising children for liver transplants since February 2002; it has been shown to provide objective ranking of potential recipients.1 Indications for transplantation are shown in Fig. 2, demonstrating a similar distribution to other paediatric liver transplant units.
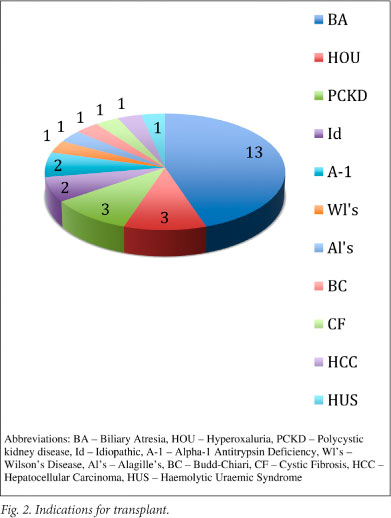
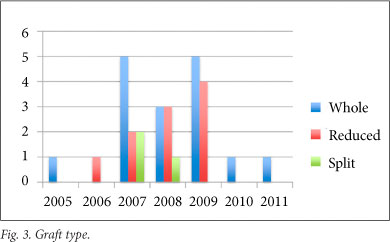
A standard multivisceral harvest technique was used for all procurement procedures and, apart from one exception where an in situ segment 2, 3, 4 split was performed, all splits and reduction procedures were performed on the back-table. Graft type (Fig. 3) included 16 whole livers, 10 reduced-size grafts and 3 split-liver segments. Seven children received combined liver-kidney transplants, 3 for primary hyperoxalurea, 3 for autosomal recessive polycystic disease, and 1 for haemolytic uraemic syndrome. Five patients received a whole liver and single kidney, 1 a whole liver with 2 kidneys en bloc (2-year-old donor), and 1 a reduced-size segment 2, 3 graft with single kidney. One liver-kidney recipient is included in our mortalities after developing both portal vein and hepatic arterial inflow occlusion within 24 hours of the transplant. Although not relevant to this series, the child who received the en bloc renal transplant developed torsion and subsequent infarction of 1 of the 2 kidneys, fortunately with excellent renal function in the remaining kidney. The 6 patients remaining in this subset are all alive and well with excellent function of both grafts.
The procedure of whole-liver procurement in paediatric donors is a combination of the initial procurement technique described by Starzl et al.,2 with the rapid flush technique.3,4 We used two implantation techniques: the classic technique with inferior vena cava replacement for whole liver grafts, and the piggyback technique with preservation of the native inferior vena cava when using reduced and split grafts.
Steroids and tacrolimus form the backbone of our immunosuppression regimen.
The series includes 3 acute peri-operative mortalities. The first, previously transplanted in Cape Town, died of fulminant sepsis; the other 2 were surgery related, 1 secondary to acute hepatic arterial thrombosis (HAT) and portal vein thrombosis (PVT), and 1 secondary to hepatic venous outflow obstruction (HVOO). Both patients were immediately re-listed but died, as suitable donors were not immediately available.
Acute surgical morbidities are listed in Fig. 4.
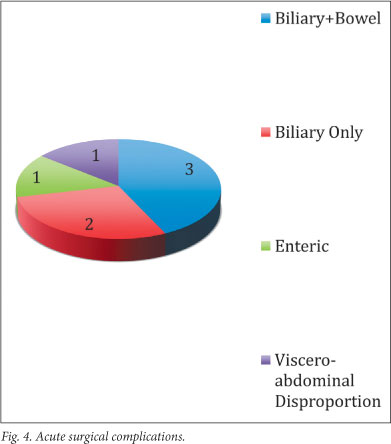
In total, there were 7 acute complications, occurring in 6 patients: 3 presented with combined biliary and enteric leaks, 2 with isolated bile leaks, 1 with an isolated colonic injury, and 1 had viscero-abdominal disproportion.
Eight long-term surgical complications, defined as occurring more than 90 days post transplant, occurred in 6 patients. These included 3 biliary strictures (1 surgically revised and 2 successfully managed with percutaneous stents), 1 symptomatic portal venous anastomotic stenosis managed by percutaneous dilatation and subsequent stenting, 1 adhesive bowel obstruction, 1 phrenic nerve palsy requiring thoracoscopic plication, 1 intrahepatic arteriovenous fistula post percutaneous liver biopsy (also successfully managed by embolisation of the arterial component) and 1 HAT. The last occurred in a reconstructed accessory right hepatic artery and was clinically insignificant. One patient with chronic rejection was subsequently re-transplanted in Cape Town.
Discussion
An estimated 2 - 5 children per million population per year require liver transplants, depending on local disease prevalence.5 The most prevalent indication for orthotopic liver transplants in children remains biliary atresia (BA).6 The primary surgical procedure in BA is the portoenterostomy as described by Morio Kasai.7 Progressive cholestasis, failure to thrive, and complications of portal hypertension (viz. gastro-intestinal bleeding, ascites and hepatopulmonary syndrome) necessitate liver transplantation in cases where biliary drainage is not effectively established, or surgery not performed. Even with successful drainage and normalisation of bilirubin, recurrent cholangitis, with subsequent development of cirrhosis and portal hypertension, may necessitate transplantation.1 Treatment of BA with sequential Kasai portoenterostomy and liver transplant occurs in most cases, with less than 20% of affected children surviving long-term with their native liver.1
Technical complications are the most important factor contributing to death in children after liver transplantation and the most common indication for re-transplantation.8 During our initial transplant experience, salient lessons learnt include: (i) a high level of vigilance is required during the multivisceral procurement procedure, particularly to identify aberrant arterial anatomy as accidental division of accessory and/or replaced vessels with subsequent reconstruction increases the risk of HAT; and (ii) that additional factors contributing to the risk of HAT during the remainder of the transplant include prolonged cold ischaemic time, intra-operative anastomotic revision, and the use of interposition grafts.9
The 'back-table' preparation of the donor liver is critically important, particularly in the paediatric setting where, in addition to whole grafts, both split and reduced-size grafts are used. The use of 'split livers'10 involves dividing the liver into 2 transplantable sections, leaving the vascular structures for the 2 portions of hepatic parenchyma intact. Alternatively, a reduced-size graft can be derived from the whole liver, discarding the remainder. Critical technical considerations are related to division of the biliary system and the hepatic veins. It is particularly important to divide the left hepatic biliary duct distal to the junction of the ducts draining segments 2 and 3, and we have found a Bake's dilator useful to identify this confluence. Failure to do so leads to these 2 ducts being divided independently, necessitating 2 separate biliary anastomoses to the Roux-en-Y limb. This increases the technical difficulty of the anastomosis and the risk of biliary anastomotic leak. The left hepatic vein should also be divided as short as possible to avoid redundancy and the potential for kinking and torsion, resulting in HVOO.
The recipient hepatectomy is equally important. Previous abdominal surgery is the most important risk factor for post-transplant bowel perforation and is especially relevant in children with BA. The risk of bowel perforation may be as high as 20% in this population.11 Familiarity with the Kasai procedure is advantageous during the liver explant, and a high index of suspicion with regard to iatrogenic enteric injuries is required, which should be identified and repaired. Therefore, we believe that paediatric surgeons should play an integral role as members of the transplant team. Finally, 'gentle' dissection of the recipient hepatic artery will prevent intimal injury and subsequent dissection, which precludes utilising this artery for the implant, unnecessarily complicating the procedure.
The paediatric liver implant requires meticulous technique, with the arterial and biliary reconstructions presenting the most challenges. Arterial anastomoses are often in the order of 3 mm in diameter, with a HAT rate of 11 - 26%.12,13 While we consider 3.5X optical magnification mandatory, it has been demonstrated that using microvascular anastomotic techniques could decrease this incidence to 1.7%.14
After mortality, the Achilles' heel of our initial experience is undoubtedly the biliary reconstruction. In the SPLIT database, biliary complications occurred in 14% of children within the first 30 days after transplantation and in 25% of children 1 - 24 months after transplant.15 Five (17%) of our patients developed biliary anastomotic leaks; all received reduced-size grafts, 4 with BA and 1 with Wilson's disease. Our learning curve is flattening, and since September 2008 we have performed 5 reduced-size grafts, all with uncomplicated biliary reconstructions. Important considerations during the construction of the biliary anastomosis are creating a tension-free Roux limb, meticulous biliary enteric anastomosis (with the use of a stent if necessary) and careful inspection for, and repair of, any explant-related serosal injuries.
Less common are hepatic and portal vein thromboses, which are more prevalent after reduced and split-liver grafts, and may present more subtly.16 Practical considerations include meticulous technique, keeping the donor hepatic vein short, and constructing the anastomosis to the common orifice of the recipient's left and middle hepatic veins, carefully everting the anastomosis.
The new paediatric liver transplant unit in Johannesburg has produced results similar to other international experiences. Perhaps the greatest attribute of our unit is that technical skills are broadly distributed among all the team individuals. Our focus on technical excellence originates from our 'parent unit', the transplant division at the University of Nebraska Medical Center, Omaha, Nebraska, who provided extensive training and support.
In the absence of related living donors, recipients rely on deceased donors as their source of organs. After the dearth of relevant paediatric specialists, organ availability remains our programme's biggest limiting factor. Our 2 surgical mortalities might have survived had they had immediate access to a second liver. The organ itself is the ultimate 'scarce resource'. Education of the public concerning the importance of organ donation remains an important focus in an attempt to produce a larger donor pool. Starzl et al. in their Pittsburgh series conclude that 'there is almost no such thing as a poor paediatric donor'.17 Of note is that South Africa subscribes to an 'opt in' as opposed to an 'opt out' donor policy.
Based on the number of our liver clinic's patients, the number of children with BA treated annually, and the numbers of paediatric transplants performed per year to date, we estimate that 10 - 20 children would be listed for transplantation annually. This demand for organs is far in excess of what the deceased donor pool can provide. Driven by this shortage of deceased donor organs, future projects include expanding our unit to include a related living donor liver transplant arm, aimed specifically at the paediatric population. Related living donation of segment 2, 3 grafts has been shown to be safe, with morbidity (10%) and mortality (0.2%) rates approaching that of RLD kidney donation.18,19 The success of paediatric living donor liver transplantation is well established worldwide. The Kyoto series - the largest in the world - has a virtually non-existent cadaveric supply.16
In general, there has been a notable shift in focus, from finding a donor organ to post-transplant quality of life in children, emphasising as near normal growth and development as possible, long-term follow-up, and preventing immunosuppression-related complications.18 This shift in focus highlights our other current challenge - the provision of specialist paediatric hepatology to our pre- and post-liver transplant patients, and paediatric intensive care in the immediate post-operative period. The lack of these two specialties currently severely curtails our paediatric liver programme, which we are desperate to remedy. This situation is an anomaly: most medical hepatobiliary units have lists of patients fulfilling the criteria for listing for transplantation, but cannot get their patients transplanted. In Johannesburg, the converse is true - we have the patients, our surgical team has overcome its learning curve in liver transplantation in the paediatric population, and we have an intense will and desire to continue, but lack the necessary support and commitment from paediatric hepatology and intensive care.
References
1. Kerkar N, Emre S. Issues unique to pediatric liver transplantation. Clin Liver Dis 2007;11(2):323-335. [ Links ]
2. Starzl TE, Hakala TR, Shaw BW Jr, Hardesty RL. Rosenthal procedure for multiple cadaveric organ procurement. Surg Gynecol Obstet 1984;158:223-230. [ Links ]
3. Starzl TE, Miller C, Broznick B, Makowka L. An improved technique for mutiple organ harvesting. Surg Gynecol Obstet 1987;165:343-348. [ Links ]
4. Nakazato PZ, Concepcion W, Bry W, et al. Total abdominal evisceration: an en bloc technique for abdominal organ harvesting. Surgery 1992;111:37-47. [ Links ]
5. Millar AJW, Spearman W, Kahn D. Paediatric liver transplantation in South Africa in 2009 (editorial). S Afr Med J 2009;99(5):308-309. [ Links ]
6. Salt A, Noble-Jamieson G, Barnes ND, et al. Liver transplantation in 100 children: Cambridge and King's College Hospital series. BMJ 1992;304:416-421. [ Links ]
7. Kasai M. Treatment of biliary atresia with special reference to hepatic porto-enterostomy and its modifications. Prog Pediatr Surg 1974;6:5-52. [ Links ]
8. Kuang AA, Rosenthal P, Roberts JP, et al. Decreased mortality from technical failure improves results in pediatric liver transplantation. Arch Surg 1996;131:887. [ Links ]
9. Stevens LH, Emond JC, Piper JB, et al. Hepatic artery thrombosis in infants. Transplantation 1992;53:396-399. [ Links ]
10. Pichlmayr R, Ringe B, Gubernatis G, Hauss J, Bunzendahl H. Transplantation of a donor liver to 2 recipients (splitting transplantation) - a new method in the further development of segmental liver transplantation. Langenbeck's Arch Chir 1988;373:127-130. [ Links ]
11. Beierle EA, Nicolette LA, Billmire DF, et al. Gastrointestinal perforation after pediatric orthotopic liver transplantation. J Pediatr Surg 1998;33:240. [ Links ]
12. Tzakis AG, Gordon RD, Shaw BWJ, Iwatsuki S, Starzl TE. Clinical presentation of hepatic arterial thrombosis after liver transplantation in the cyclosporine era. Transplantation 1985;40:667-671. [ Links ]
13. Mazzaferro V, Esquivel CO, Makowka L, et al. Factors responsible for hepatic arterial thrombosis after pediatric liver transplantation. Transplant Proc 1989;21:2466. [ Links ]
14. Inomoto T, Nishizawa F, Sasaki H, et al. Experiences of 120 microsurgical reconstructions of hepatic artery in living related liver transplantation. Surgery 1996;119:20. [ Links ]
15. SPLIT Research Group. Studies of pediatric liver transplantation (SPLIT): year 2000 outcomes. Transplantation 2001;72(3):463-476. [ Links ]
16. Mc Diarmid S. Liver transplantation: The pediatric challenge. Clin Liver Dis 2000;4:4. [ Links ]
17. Starzl T. Pediatric liver transplantation. Transplant Proc 1987;19(4):3230-3235. [ Links ]
18. Spada M, Riva S, Maggiore G, Cintorino D, Gridelli B. Pediatric liver transplantation. World J Gastroenterol 2009;15(6):648. [ Links ]
19. Broelsch CE, Whitington PF, Emond JC, et al. Liver transplantation in children from related living donors: surgical techniques and results. Ann Surg 1991;214(4):428-437. [ Links ]
Accepted 22 August 2011.
Corresponding author: J Loveland (loveland@wol.co.za)














