Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.102 n.3 Pretoria Mar. 2012
GUIDELINE
Paediatric anticoagulation guidelines
E SchapkaitzI; G G ShermanII; B F JacobsonIII; S HaasIV; H R BullerV; V DaviesVI; H DiarVII; M PetersVIII; M ShuttleworthIX; S VelaphiX
IMB BCh, FCPath (Haem), MMed (Haem), National Health Laboratory Services, and Department of Haematology, University of the Witwatersrand, Johannesburg
IIMB BCh, MMed (Haem), DCH (SA), DTM&H, PhD, National Health Laboratory Services, and Department of Haematology, University of the Witwatersrand, Johannesburg
IIIMB ChB, MMed (Haem), FRCS (Glasgow), PhD, National Health Laboratory Services, and Department of Haematology, University of the Witwatersrand, Johannesburg
IVMD, PhD, Institute for Experimental Oncology and Therapy Research, Technical University of Munich, Germany
VMD, PhD, Department of Vascular Medicine, Academic Medical Centre, University of Amsterdam, The Netherlands
VIMD, Department of Vascular Medicine, Academic Medical Centre, University of Amsterdam, The Netherlands
VIIMB BCh, FC Paeds (SA), Department of Paediatrics and Child Health, University of the Witwatersrand, Johannesburg
VIIIMB BCh, FC Paeds (SA), Cert Neonatol (SA), Department of Paediatrics and Child Health, University of the Witwatersrand, Johannesburg
IXMB hB, MMed, FC Paeds (SA), Department of Paediatrics and Child Health, University of the Witwatersrand, Johannesburg
XBSc (Hons) MB ChB, MMed (Haem), Department of Haematology, Red Cross Children's Hospital, University of Cape Town
ABSTRACT
BACKGROUND: Recent progress has been made in the understanding of venous thrombo-embolism (VTE) in children and neonates; however, indications for laboratory investigations and therapeutic interventions are not well defined.
METHOD: The Southern African Society of Thrombosis and Haemostasis reviewed available literature and comprehensive evidence-based guidelines for paediatric antithrombotic therapy. A draft document was produced and revised by consensus agreement. The guidelines were adjudicated by independent international experts to avoid local bias.
RESULTS AND CONCLUSION: We present concise, practical guidelines for the clinical management and laboratory investigation of VTE in children and neonates. Recommendations reflect current best practice which will hopefully lead to improved anticoagulation practice in this age group.
1. Introduction
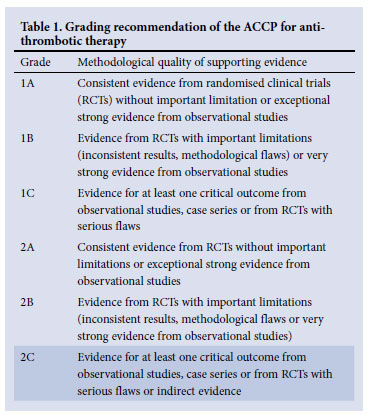
These guidelines for the clinical management and laboratory investigation of venous thrombo-embolism (VTE) in neonates and children reflect current best practice. Recommendations presented are in accordance with the more comprehensive evidence-based guidelines for paediatric antithrombotic therapy, including the American College of Chest Physicians (ACCP) guidelines, the United Kingdom paediatric stroke guidelines and the American Heart Association guidelines for paediatric stroke.1-6 Many of the recommendations are Grade 2C, indicating the lack of strong evidence (Table 1). The most current paediatric recommendations are extrapolated from adult trials; therefore, management should be individualised in consultation with a haematologist with appropriate consideration for the risk/ benefit ratio of each patient. Drug recommendations are based on Medicines Control Council registration at the time of publication.
2. Antithrombotic therapy in paediatric patients
The aim of primary anticoagulation is to prevent thrombus propagation and to achieve clot resolution. Antithrombotic therapy in paediatric patients cannot be extrapolated directly from adult recommendations. Features specific to the paediatric age group include:
• The aetiology of VTE: the presence of a central venous catheter (CVC) is the most important risk factor for the development of thrombosis in neonates and children. Additional documented risk factors include sepsis, perinatal hypoxia, dehydration, maternal diabetes and polycythaemia in neonates, and congenital heart disease, cancer, chemotherapy, protein-losing states, total parenteral nutrition (TPN) and trauma/surgery in children.
• The haemostatic system is a dynamic and evolving system that matures in late adolescence. This affects the frequency of VTEs in children and the response to antithrombotic therapies. VTE in the paediatric age group is rare compared with adults, with a peak incidence in the neonatal period and adolescence.
• Limited vascular access.
The management of neonates and children should also be considered separately, because they differ in terms of the aetiologies of VTE and the pharmacokinetics of antithrombotic therapies.
3. Specific indications forantithrombotic therapy
• Many of the recommendations are Grade 2C, indicating that most of the data are based on observational studies.
• Before the initiation of antithrombotic therapy, baseline testing of partial thromboplastin time (PTT), prothrombin time (PT)/ international normalised ratio (INR) and platelet count should be performed.
• Intracranial bleeding should be excluded, especially in premature infants, by cranial ultrasound.
• The duration of antithrombotic therapy needs to be individualised according to the patient's thrombo-embolic and bleeding risk; raised D-dimers >0.5 mg/l and residual thrombus after 3 -6 months of antithrombotic therapy are considered to be risk factors for recurrent VTE.
3.1 Neonatal VTE
• CVC-related VTEs account for two-thirds of VTE.
• Immediate removal of the catheter, if possible, is suggested.
• The optimal duration of anticoagulation remains undefined, but short-term therapy with low molecular weight heparin (LMWH) or unfractionated heparin (UFH) for 10 - 14 days is suggested, with objective radiological monitoring (echocardiography or ultrasound) performed both during and after the completion of anticoagulant therapy.
• If there is evidence of a new or extending thrombus following completion of initial therapy, anticoagulation should be recommenced.
• Thrombolytic therapy for neonatal VTE is not recommended.
3.2 Neonatal renal vein thrombosis (RVT)
• It is suggested that anticoagulation with LMWH or UFH be given for 3 months for unilateral RVT.
• In addition to anticoagulation, thrombolytic therapy is suggested for bilateral RVT with renal failure.
3.3 Neonatal cerebral sinovenous thrombosis (CSVT)
• Anticoagulation with LMWH or UFH is suggested for at least 6 weeks and up to 3 months for neonatal CSVT if there is no significant haemorrhage. Neonates recanalise at a faster rate than older children and radiological assessment for recanalisation can be performed at 6 weeks. If recanalisation is complete, anticoagulation therapy can be stopped.
• Radiological monitoring of the thrombosis at 5 - 7 days is recommended for neonatal CSVT with significant haemorrhage. Anticoagulation is suggested if thrombus extension is noted.
3.4 Purpura fulminans
• For neonates with homozygous protein C or S deficiency, the administration of fresh frozen plasma (10 - 20 ml/kg) every 6 - 12 hours is recommended. Replacement therapy is recommended following 6 -8 weeks, to facilitate resolution of the clinical lesions.
• Long-term anticoagulation with LMWH or warfarin is suggested.
3.5 VTE in children
• Initial anticoagulation with LMWH or UFH is recommended for at least 5 - 10 days, followed by warfarin or LMWH.
• Maintenance warfarin therapy can be started on day 1 or 2 of heparin therapy.
• Routine use of thrombolytic therapy is not recommended, unless there is major vessel occlusion causing critical compromise of limbs or organs.
• Suggested duration of anticoagulation:
• CVC-related VTE: at least 3 months with objective radiological monitoring (echocardiography or ultrasound) performed both during and after the completion of anticoagulant therapy
• Idiopathic VTE: at least 6 months
• Recurrent idiopathic VTE: long-term anticoagulation, possibly life-long
• Secondary VTE: until the risk factor (e.g. nephrotic syndrome or L-asparaginase chemotherapy) has resolved for at least 3 months.
3.6 CSVT in children
• Anticoagulation with LMWH or UFH for at least 5 - 10 days, followed by warfarin or LMWH is suggested for a minimum of 3 months up to 6 months for CSVT in children if there is no significant haemorrhage.
• Radiological assessment for recanalisation can be performed at 3 months. If recanalisation is complete, anticoagulation therapy can be stopped.
• Radiological monitoring of the thrombosis at 5 - 7 days is recommended for CSVT with significant haemorrhage; anticoagulation is suggested if thrombus extension is noted.
3.7 VTE associated with cancer and chemotherapy
• Routine antithrombotic prophylaxis is not recommended in paediatric patients with cancer due to the lack of evidence and the potential for increased bleeding.
3.8 Children with congenital heart defects post surgery
• Intra- and/or postoperative therapy with UFH is suggested, followed by either aspirin (1 - 5 mg/kg/dose) or anticoagulation with LMWH or warfarin.
4. Antithrombotic drugs
Antithrombotic drugs available for the management of VTE in the paediatric population include:
• LMWH
• UFH
• Vitamin K antagonists.
The guidelines below pertain to the use of LMWH (enoxaparin), UFH and warfarin.
4.1 LMWH (enoxaparin)
LMWH offers definite advantages over UFH because of its superior bioavailability, longer half-life, dose-dependent clearance (resulting in a more predictable anticoagulant response), subcutaneous administration and minimal monitoring requirements. The use of LMWH should be avoided in renal insufficiency.
4.1.1 LMWH dose
The recommended doses for enoxaparin are as follows:
Treatment dose:
• Administer enoxaparin at 1.5 mg/kg/dose subcutaneously (SC) per 12 hours for neonates aged <2 months and <5 kg*.
• Administer enoxaparin at 1 mg/kg/dose SC per 12 hours for children aged 2 months - 16 years.
Prophylactic dose:
• Administer enoxaparin at 0.75 mg/kg/dose SC per 12 hours for neonates aged <2 months.
• Administer enoxaparin at 0.5 mg/kg/dose SC per 12 hours for children aged 2 months - 16 years.
Available enoxaparin preparations often need to be diluted with sterile water to a concentration of 20 mg/ml to administer the small doses required. The preparation must be refrigerated after opening.
4.1.2 Monitoring of therapy
• Avoid other antiplatelet drugs, intramuscular (IM) injections and arterial punctures during anticoagulation.
• Monitoring of LMWH is recommended in children, especially in neonates. In sick preterm neonates, the initiation dose of 2.0 mg/kg/ dose SC per 12 hours may be insufficient to achieve a therapeutic level. Close monitoring is advised in the initial treatment phase to avoid delays in achieving adequate anticoagulation.
• Anticoagulant activity is measured using an anti-Xa activity assay.
• Anti-Xa monitoring should be performed in consultation with a haematologist. Testing is currently available at the Charlotte Maxeke Johannesburg Academic Hospital Coagulation Laboratory (+27 (0)11 489 8471/8534); the Red Cross Children's Hospital Haematology Laboratory, Western Cape (+27 (0)21 658 5203/5204) as well as most private laboratories.
• A 5 ml citrate tube (or 2 x 1 ml paediatric citrate tubes) is required for the assay. Avoid sampling blood from indwelling lines. Samples must reach the reference laboratory (or local laboratory for shipping) within 1 hour.
• Samples must be drawn 3 - 4 hours after the last LMWH subcutaneous injection and repeated until a therapeutic anti-Xa level is achieved.
• Once therapeutic levels are achieved, continued regular monitoring and dose adjustments for changes with growth are recommended.
Target anti-Xa levels:
• Prophylaxis target: 0.2 - 0.4 anti-Xa U/ml
• Therapeutic target: 0.5 - 1.0 anti-Xa U/ml.
Dose adjustments should be performed according to the nomogram in Table 2. If an inadequate response is achieved, the following should be considered:
Low anti-Xa level:
• Timing of specimen draw
• Heparin resistance secondary to antithrombin deficiency, e.g. neonates, sepsis, consumption due to large thrombus
• Dilution and administration of LMWH
• Dose of LMWH omitted
• Weight gain.
High anti-Xa level:
• Heparinised syringe
• Weight loss
• Renal dysfunction.
4.1.3 Management of bleeding
• LMWH (plasma half-life of 3 - 6 hours) should be discontinued, as well as any other agents that may contribute to bleeding .
• Measurement of anti-Xa levels may be indicated.
• One intravenous dose (IV) of protamine sulphate can neutralise about 75% of the anti-Xa activity.
• 1 mg of protamine sulphate IV should be administered per 1 mg (equivalent to 100 anti-Xa units) of enoxaparin given in the last dose over 10 minutes.
• If the patient continues to bleed, a repeat dose of 0.5 mg protamine sulphate IV per 1 mg of enoxaparin, up to a maximum of 3 doses, can be administered.
4.2 UFH
UFH may be preferentially used in children and neonates who are at risk of bleeding, have poor renal function or require surgery, in view of the short half-life, minimal renal excretion and rapid reversal by protamine sulphate.
4.2.1 UFH dose
The recommended doses for UFH are as follows:
Treatment dose:
• Administer a bolus of 25 - 50 U/kg IV over 10 minutes and an initial maintenance dose of 15 U/kg/h IV as a continuous infusion for preterm neonates.
• Administer a bolus of 75 -100 U/kg IV over 10 minutes and an initial maintenance dose of 28 U/kg/h IV as a continuous infusion for term neonates.
• Administer a bolus of 75 -100 U/kg IV over 10 minutes and an initial maintenance dose of 20 U/kg/h IV as a continuous infusion for children.
• Neonates need a higher maintenance dose per body weight compared with older children.
Prophylactic dose:
• Administer UFH at 10 U/kg/h IV as a continuous infusion.
4.2.2 Monitoring of therapy
• Avoid other antiplatelet drugs, IM injections and arterial punctures during anticoagulation.
• The patient's platelet count should be monitored on initiation of UFH and 7 days later while on therapy to assess for heparininduced thrombocytopenia (HIT). If the patient has been exposed to UFH in the last 100 days, a repeat platelet count is indicated within 24 hours of starting UFH. The risk of HIT is greatest after 5 -10 days of UFH therapy, but still rare in children. HIT should be considered when all other causes of thrombocytopenia have been ruled out. UFH should be stopped and other antithrombotic drugs should be started, e.g. Fondaparinux 0.1mg/kg SC daily.
• Anticoagulant activity is measured using a PTT test (or anti-Xa heparin assay).
• A 5 ml citrate tube or 1 ml paediatric citrate tube is required for the assay. Avoid sampling blood from indwelling lines.
Target levels:
• PTT: 60 - 85 seconds or 2 - 3 times the baseline value (if normal for age).
The PTT should be monitored 4 hours post bolus injection and the continuous IV dose adjusted according to the result (Table 3).
4.2.3 Management of bleeding
• Bleeding can usually be controlled by stopping the infusion (plasma half-life of 25 minutes in neonates and 1 - 2 hours in children).
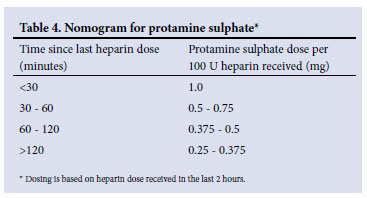
• If bleeding is life-threatening, immediate reversal of UFH activity can be achieved by administration of protamine sulphate (Table 4).
• Administer protamine sulphate IV over 10 minutes at a concentration of 10 mg/ml. Rapid infusion can cause hypotension.
• Following IV administration, neutralisation occurs within 5 minutes.
• The maximum dose of protamine sulphate that can be administered is 50 mg.
4.3 Warfarin
Warfarin is not recommended in neonates. Warfarin administration in children is complicated by the need for frequent monitoring and dose adjustments due to diet interactions (low vitamin K in breast milk or high vitamin K in TPN and formula), intercurrent illness and drugs metabolised by cytochrome P450 enzymes. Warfarin is teratogenic and adolescent females should receive appropriate counselling.
4.3.1 Warfarin dose
The recommended dose for warfarin is as follows:
Treatment dose:
• Warfarin should always be preceded by heparin therapy.
• Initiate warfarin at a dose of 0.2 mg/kg to a maximum of 10 mg on day 1 or 2 of heparin therapy; if the patient has liver dysfunction or has had a Fontan procedure start at 0.1 mg/kg.
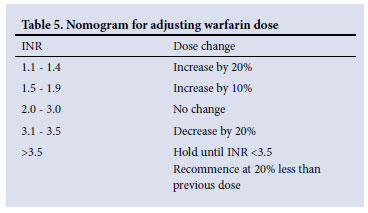
• Doses can be adjusted by small increments of 0.5 mg/dose (Table 5).
4.3.2 Monitoring of therapy
• Avoid other antiplatelet drugs, IM injections and arterial punctures during anticoagulation.
• Anticoagulant activity is monitored using an INR test.
• A 5 ml citrate tube or 1 ml paediatric citrate tube is required for the assay, with avoidance of sampling blood from indwelling lines
• The INR should be monitored initially after 3 -5 days and then daily until a therapeutic range is achieved; repeat INR is indicated 5 - 7 days later.
• Heparin is not discontinued until the INR is therapeutic for 2 consecutive days.
• Once stable, the INR should be monitored a minimum of once per month; most children require monitoring every 2 weeks.
• Fluctuations of the INR outside the desired target range should be investigated.
Target levels:
• The target INR is 3.0 (range 2.5 - 3.5) for patients with valvular heart disease, antiphospholipid syndrome and warfarin failure.
• The target INR is 2.5 (range 2.0 - 3.0) for other indications.
4.3.3 Management of bleeding
• Bleeding associated with warfarin therapy can be severe because of its long plasma half-life of 40 hours.
• If bleeding is significant, warfarin activity can be reversed immediately by administration of fresh frozen plasma (10 - 20 ml/kg IV) or vitamin K (0.5 - 1 mg) per os (PO) or slow IV.
4.4 Systemic thrombolytic therapy
Thrombolytic therapy is not recommended in neonates. It is contraindicated if there is active bleeding, general surgery in the previous 10 days or neurosurgery in the previous 3 weeks. Tissue plasminogen activator (t-PA) is the preferred drug in children.
4.4.1 t-PA dose
The recommended dose for t-PA is as follows:
Treatment dose:
• Start infusion at a rate of 0.5 mg/kg/h IV for 6 hours.
• Start heparin without a bolus at 20 U/kg/h IV during the t-PA infusion.
• Re-evaluate clinically and radiographically after 6 hours of t-PA infusion; if there is no response, a repeat t-PA infusion can be considered.
4.4.2 Monitoring of therapy
• Avoid other antiplatelet drugs, IM injections and arterial punctures during anticoagulation.
• Monitor full blood count (FBC), PT, PTT, fibrinogen and D-dimer levels 4 - 6 hourly.
• Adjust the continuous IV heparin dose according to the PTT result (Table 3).
Target levels:
•Fibrinogen >1.0 g/l
•Platelets >100 x 109/l, and
•PTT 60 - 85 seconds or 2 - 3 times the baseline value
4.4.3 Management of bleeding
• Mild bleeding from a puncture site can be treated supportively and with local pressure.
• If bleeding is significant, stop the t-PA infusion and administer cryoprecipitate at 1 U/5 kg and other blood products as indicated.
• Protamine sulphate may be required to reverse the UFH activity (Table 4).
5. Thrombophilia testing
VTE is often a multifactorial disease, and acquired risk factors in sick children often play a more important role than inherited risk factors.
5.1 Recommended investigations
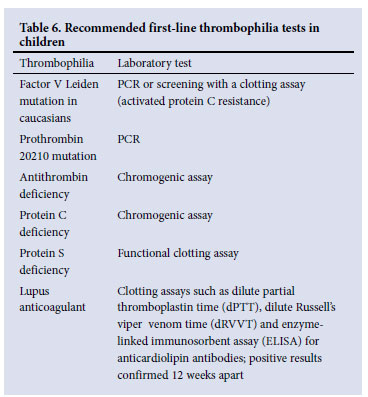
• Recommended first-line thrombophilia tests include conditions that are most prevalent in children (Table 6).
• Results must be interpreted in conjunction with age-adjusted reference ranges; abnormal results should be confirmed with repeat testing.
• The results of thrombophilia testing rarely influence early management decisions.
• Testing is best done after 6 months of age, in the non-acute setting, and at least 2 weeks after discontinuation of anticoagulation therapy, because the results are altered by the acute event and by anticoagulant therapy.
• Exceptions include antithrombin levels (in the setting of heparin resistance), antiphospholipid antibodies (in the setting of maternal antiphospholipid syndrome), protein C and protein S in neonates presenting with purpura fulminans, and polymersase chain reaction (PCR) testing for Factor V Leiden and the prothrombin gene mutations.
5.2 Thrombophilia testing
• Thrombophilia testing should be strongly considered in adolescents with spontaneous VTE; this group has the highest prevalence of inherited thrombophilia.
• Thrombophilia testing for protein C, protein S and antithrombin should be strongly considered in neonates presenting with purpura fulminans, ischaemic skin lesions or extensive thrombosis.
• Thrombophilia testing should be considered in neonates and children with non-catheter-related VTE.
• Thrombophilia testing is not recommended with catheter-related VTE, but can be considered in neonates and children with recurrent catheter-related VTEs.
• Concomitant screening of both parents, with appropriate counselling, is recommended.
References
1. Monagle P, Chalmers E, Chan A, et al. Antithrombotic therapy in neonates and children: American College of Chest Physicians evidence-based clinical practice guidelines (8th ed). Chest 2008;133:887S-968S. [ Links ]
2. The Paediatric Stroke Working Group. Stroke in childhood: Clinical guidelines for diagnosis, management and rehabilitation. London: Royal College of Physicians, 2004. http://bookshop.rcplondon.ac.uk/contents/f98c6540-a541-4bed-837d-ef293ac458bf.pdf (accessed 25 November 2011). [ Links ]
3. Roach ES, Golomb MR, Adams R, et al. Management of stroke in infants and children. Stroke 2008;39:2644-2691. [ Links ]
4. Manco-Johnson MJ, Grabowski EF, Hellgreen M, et al. Laboratory testing for thrombophilia in pediatric patients. Thromb Haemost 2002;88:155-156. [ Links ]
5. Andrew M, Paes B, Johnston M. Development of the hemostatic system in the neonate and young infant. Am J Pediatr Hematol Oncol 1990;12:95-104. [ Links ]
6. Malowany JI, Monagle P, Knoppert DC, et al. Enoxaparin for neonatal thrombosis: a call for a higher dose for neonates. Thromb Res 2008;122:826-830. [ Links ]
Accepted 25 November 2011
on behalf of the South African Society of Thrombosis and Haemostasis
Corresponding author: E Schapkaitz (elise.schapkaitz@nhls.ac.za)
* Neonates need a higher dose per body weight compared with older children because of their larger volume of distribution, accelerated drug clearance and lower plasma antithrombin levels. A higher initiation dose of enoxaparin at 1.7 mg/kg/dose SC per 12 hours for term neonates and 2.0 mg/kg/dose SC per 12 hours for preterm neonates aged <28 weeks may be required to achieve a therapeutic anti-Xa level.














