Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.101 n.12 Pretoria Dec. 2011
ORIGINAL ARTICLES
Safety and efficacy of procedural sedation and analgesia (PSA) conducted by medical officers in a level 1 hospital in Cape Town
G Wenzel-SmithI; B SchweitzerII
IStateExamMed (Aachen), MRCGP (UK), DA (SA), FamMedReg. Department of Family Medicine, University of Cape Town
IIMB BCh, MPraxMed, DA (SA). Department of Family Medicine, University of Cape Town
ABSTRACT
OBJECTIVES: To study the efficacy and safety of procedural sedation and analgesia (PSA) administered by medical officers (MOs) without formal anaesthetic training.
METHODS: A retrospective descriptive study in the Emergency Department (ED) of False Bay Hospital (FBH), situated in the southern suburbs of the Cape Town Metro Health District. The study included all patients who received PSA at FBH between 1 March 2007 and 31 August 2009. Variables recorded included age, gender, physical status as determined by the American Society of Anesthesiologists (ASA status), procedure, fasting and intoxication status, PSA medications, adverse effects, rescue manoeuvres performed, if any, and time to discharge. Analysis was largely descriptive and clinical and demographic data are presented as means (standard deviations), medians, ranges and proportions as appropriate. Success of sedation and incidence of adverse effects are presented as proportions.
RESULTS: Of 166 patients, 140 (84.3%) showed a good level of sedation, 14 (8.4%) were inadequately sedated, 5 (3%) were too deeply sedated but showed no signs of respiratory compromise, and 7 (4.2%) developed respiratory side-effects. Respiratory complications were treated with simple airway manoeuvres; no patient required intubation or experienced respiratory problems after waking up. There was no significant difference in the risk of adverse effects between the fasted and non-fasted groups. Mildly intoxicated patients who received PSA were at a higher risk of adverse effects.
CONCLUSION: PSA can be administered safely by medical officers. Future research should expand on PSA research in this setting and focus on safety and patient satisfaction.
Procedural sedation and analgesia (PSA) is a skill commonly required when dealing with patients in the emergency department (ED). Typical procedures performed under PSA in the ED or minor theatre setting are reduction of fractures and common dislocations, incision and drainage of abscesses, laceration repair in children, foreign body removal, and evacuation of retained products of conception (RPOC).
Insufficient analgesia is associated with unwanted physiological and psychological side-effects, including increased sympathetic outflow, peripheral vascular resistance, myocardial oxygen consumption, production of carbon dioxide, hypercoagulability, decreased gastric motility, decreased immune function, and the subsequent development of chronic pain.1-3
Considerable research has proved the safety and efficacy of PSA when administered by emergency physicians in ED units around the world. There are few studies on PSA administration by nonspecialists in the public health sector in South Africa.4 Over 80% of the population is serviced by the state-funded public sector hospitals, which are often overcrowded and under-resourced.5
The South African Department of Health6 considers that it is the responsibility of the medical officer (MO) or family physician to care for patients in the ED and administer PSA.7 Our objectives were to study the safety and efficacy of PSA when provided by MOs in a South African peri-urban district hospital, and the influence of fasting status and intoxication on sedation outcome and adverse effect rate.
Setting
False Bay Hospital is situated in Fish Hoek in the southern suburbs of the Cape Town Metro Health District. It is attended by a diverse patient population, including those from a very low socio-economic background and wealthier patients belonging to medical aids. It is a district hospital with 75 inpatient beds, 2 operating theatres, an outpatient department, and an ED with an annual head count of about 14 000 patients. When the study was conducted, the ED was staffed by MOs with post-graduation working experience between 5 and 12 years and no formal training in emergency medicine or anaesthetics.
Owing to lack of trained staff the hospital had no PSA service before commencement of the study, a situation mirrored in many of South Africa's smaller primary health care facilities. Procedures in the ED were either conducted without PSA or referred to a secondary hospital.
Before 2007, dilatation and curettage (D&C) of the uterus after incomplete or missed abortion was only undertaken on selected days when a local general practitioner (GP) with a diploma in anaesthetics was available. When the GP was unavailable, patients requiring a D&C were referred to a secondary hospital. One of the authors (GW-S) attended an accredited 2-year diploma course in conscious sedation at the University of the Western Cape and subsequently provided in-house training to interested medical and nursing staff.
PSA guidelines8-10 were circulated to staff involved in PSA and adhered to.
Methods
This was a consecutive case series with retrospective evaluation of records of all patients requiring PSA who presented to the ED of False Bay Hospital between 1 March 2007 and 30 August 2009. Patients who received medications for the purpose of PSA, orally, by inhalation or intravenously, were included. Patients who had incomplete abortions and were treated in the minor operating theatre of the same hospital with a D&C under PSA were included. Patients who received any drugs usually administered for PSA for endotracheal intubation, seizure control or analgesia without an associated procedure were excluded.
The MO in the ED was responsible for selecting patients deemed suitable for PSA at a level 1 facility, and for the choice of agents used for PSA. All health care staff participating in PSA had in-house training in PSA medication, and standardised guidelines8-10 were followed.
Drugs were used at doses suitable for PSA as opposed to anaesthetic doses. Propofol was mixed into a 1:1 solution with ketamine, commenced at a dosage of 0.2 mg/kg for each drug and titrated to the desired effect in 2 ml increments (1 ml of the mixed solution contained 5 mg each of propofol and ketamine). Ketamine, when used alone, was used at a starting dose of 0.5 mg/kg and slowly titrated in increments of 0.2 mg/kg. Drug choices were up to the attending MO.
All MOs administering PSA had attended ACLS (advanced cardiac life support), ATLS (advanced trauma life support) and PALS (paediatric advanced life support) courses.
Patients selected for PSA at False Bay Hospital were generally 'healthy', meaning ASA (American Society of Anesthesiologists classification) status 1 or 2, or stable ASA 3 patients, with no psychiatric disease. Fasting status and intoxication with alcohol were evaluated and the decision to proceed with or defer the procedure was made on a case-to-case basis by the responsible MO. Patients who attended the ED for procedures other than D&Cs were not routinely fasted, 10 patients were mildly intoxicated but found suitable for PSA, and one procedure was deferred because of the patient's level of intoxication. All the D&C patients were fasted.
Informed consent was obtained for the procedure and sedation. Ethics approval for the study was obtained from the UCT Ethics Committee.
Each procedural sedation event was recorded on a standardised anaesthetic record sheet. Recorded variables included age, sex, ASA status, presenting problem, fasting status, clinical impression of intoxication, PSA medications and dosages used, adverse effects, rescue manoeuvres performed, if any, and time to discharge if discharged or to other disposal of the patient. Patients were monitored throughout the procedure with continuous pulse oximetry, and heart rate and blood pressure were measured before and at 2-minute intervals after commencement of the procedure. Readiness for discharge was determined in accordance with an Aldrete score of 9/10.
Adverse events were categorised as follows: (i) apnoea - no respiratory effort for >20 seconds; (ii) desaturation - oxygen (O2) saturation <93%; (iii) airway manoeuvre required (bag/valve ventilation); (iv) bradycardia - heart rate <50/min; (v) inadequate sedation ± cancellation of procedure due to failure of PSA; (vi) vomiting/nausea; and (vii) hallucinations.
Results
Data were entered into an Excel spreadsheet. Data analysis is largely descriptive and clinical and demographic data are presented as means (standard deviations (SDs)), medians, ranges, and proportions as appropriate. Success of sedation and incidence of adverse effects are presented as proportions.
The mean age was 23 years (SD 17.98). The oldest patient was 88 years and the youngest patient 3 months old. Table I sets out the frequency of other demographic variables.
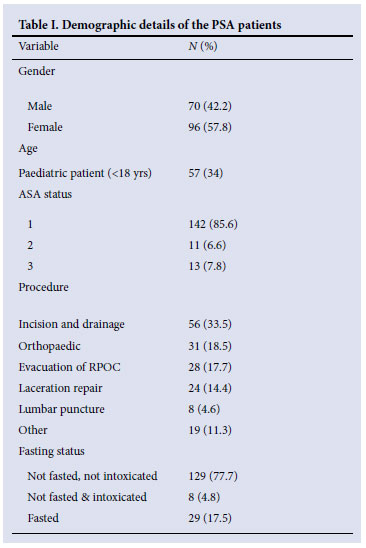
The intended procedures could be completed in 165 (99.4%) of 166 patients; 9 (54.42%) experienced minor adverse effects, with no intubation required and no long-term problems as judged by review of patient records and of mortality and morbidity meetings for the time span concerned.
Table II contains the breakdown of adverse events for the PSA patients. There was no statistically significant difference between complication rates for male and female patients (p>0.05).
There was a statistical difference in age (p=0.0024) between the patients who experienced complications and those who did not. Patients who experienced side-effects from their treatment were older on average, with a median age of 40 years versus a median age of 22 years for those who did not experience side-effects. The youngest patient who experienced an adverse effect was 19 years old.
The numbers were too low for statistical analysis of the different medication groups for complications. However, there was a trend towards a higher complication rate with addition of propofol and use of multiple sedation drugs.
The numbers were too small for statistically significant conclusions concerning adverse events in fasted versus non-fasted versus intoxicated patients, but there was a tendency for intoxicated patients to develop complications, while there was little difference in the adverse effect rate between fasted and non-fasted patients (Table III).
Of the patients 143 were discharged after their procedure; the remaining 23 required admission or referral for further treatment. None of the admissions or referrals was related to PSA. The mean discharge time was 73 minutes (SD 60.3), with the lowest time to discharge being 10 minutes and the highest 222 minutes.
Discussion
The South African Department of Health guidelines place the provision of PSA under the responsibility of level 1 hospitals.7 This research was conducted in such a hospital, staffed by MOs, to determine the outcome of PSA.
The adverse effect rate (complication rate) overall was low and in keeping with reports from other countries.11-14
An unexpected research outcome was detection of a significant difference in side-effects in relation to age. The median age of the patients who experienced complications was 40 years versus 23 years in those who did not (two-sample Wilcoxon rank-sum test: p=0.0024). There may be two reasons for this. Firstly, advanced age is known to be a risk factor for anaesthetic complications and complications of PSA.15 Secondly, most paediatric patients received fewer and lower doses of PSA drugs; 22 (38.6%) of all children presented for laceration repair, while the more painful incision and drainage of abscess was the most common procedure in adults (41.3%). Laceration repair requires 'lighter' anxiolytic medication in order for the child to allow infiltration of the affected skin for pain relief. As such, side-effects relating to the use of PSA medication would be less likely.
We also suspect that attending MOs may in general be more 'careful' when sedating children, and hesitant to prescribe larger doses or combinations of drugs. We did not examine how far this might have led to under-dosing of the children involved, which might be an interesting topic for future research.
Comparison of the PSA medication that children and adults received showed that while 55 (50.5%) of adults received ketamine and midazolam, 42 (75.4%) of children had single agents (N2O, midazolam, ketamine or an opiate) for PSA.
A trend was found towards a higher risk of complications with multi-drug regimens and addition of propofol. This is expected, as propofol has a respiratory depressant effect16 and a combination of benzodiazepines and opiates can cause respiratory side-effects.17 Future use of this medication combination for PSA will be reviewed. Intoxicated patients were also at an increased risk of adverse effects.
Alcohol ingestion more than doubled the complication rate in our series. While this finding is not statistically significant owing to small patient numbers, it indicates that guidelines, especially regarding administration of PSA to intoxicated patients, were not always closely followed. While it may be argued that later reduction of a dislocated joint is more difficult, and reduction should therefore be done as soon as possible (5 of 8 intoxicated patients had dislocated joints), this is not true for incision and drainage of an abscess (2 of 8 intoxicated patients received PSA for drainage of an abscess). Such procedures should be done electively with a fasted and sober patient. Of the 8 intoxicated patients one suffered a short spell of apnoea, having received a combination of ketamine and midazolam for PSA after being given morphine for pain relief by ambulance staff en route to the hospital for a painful dislocated shoulder. In this case the combination of alcohol, morphine and midazolam (with the added ketamine) predisposed the patient to adverse effects.
The complication rates between fasted and non-fasted patients differed little. In fact, fasted patients had a slightly higher complication rate (6.9%) than those who had not fasted (4.7%). These findings were not statistically significant owing to low patient numbers, as well as a low overall number of complications. While fasting rules have been propagated for PSA as an extension of anaesthesia practice, in recent years applicability of nil per os guidelines for PSA has been questioned.18,19
Limitations
While this study was a moderately powered retrospective case review, it lacks the numbers to uncover a serious adverse event. The expected numbers of sedation-induced deaths or permanent neurological injuries are small, in the order of one in tens of thousands. Patient numbers in the order of 50 000 would be needed to investigate these events.
Rating of a 'successful' procedure is a problem, especially from a patient-centred family physician approach. Rates of sedative failure have been reported to be as low as 1 - 3%20, 21 and as high as 10 -20%.22,23 While the success rate depends on the setting (including the drugs used, the provider, psychological support, and presence or absence of a parent in PSA of children), it also depends on the definition used for successful sedation. In this study PSA was judged to be successful if the procedure could be completed. The condition of the patient was not described further. This study would therefore have described a procedure done on a child who received PSA but screamed during the procedure and then slept deeply afterwards as a 'success' when in reality it was not.
A patient satisfaction questionnaire is probably the only way to ascertain true success of a procedure in a holistic, patient-centred way, and more research on PSA should be planned using this approach.
Some might feel that lack of a standardised drug regimen was a limitation. However, the research question was not to prove superiority of a certain drug for providing PSA, but to prove that PSA can be administered safely by non-specialised but trained medical staff.
Conclusion
PSA can be administered safely by MOs in district hospitals. Future research should expand on PSA research in this setting, focusing not just on safety but also on patient satisfaction with PSA.
Newly qualified doctors in South Africa are likely to spend their first few years in district level care. Safe provision of PSA should therefore be taught to more doctors (as a postgraduate course) and even to undergraduate medical students.
Adherence to PSA guidelines, knowledge of drugs and basic airway management are of the upmost importance.
Most importantly, the relief and avoidance of pain is central to our role as humane professionals providing quality health care.
References
1. Beilin B, Shavit Y, Trabekin E, et al. The effects of post-operative pain management on immune response to surgery. Anesth Analg 2003;97(3):822-827. [ Links ]
2. Charmandari E, Kino T, Souvatzoglou E, et al. Pediatric stress: hormonal mediators and human development. Horm Res 2003;59(4):161-179. [ Links ]
3. Koga C, Itoh K, Aoki M, et al. Anxiety and pain suppress the natural killer cell activity in oral surgery outpatients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2001;91(6):654-658. [ Links ]
4. Hodkinson PW, James MFM, Wallis LA. Emergency department procedural sedation practice in Cape Town, South Africa. Int J Emerg Med 2009;2(2):91-97. [ Links ]
5. Matsebula T, Willie M. Private hospitals. In: Harrison S, Bhana R, Ntuli A, eds. South African Health Review. Durban: Health Systems Trust, 2007. [ Links ]
6. Department of Health. L1/L2/L3 Acute Hospital Packages of Care. August 2009. Department of Health, Provincial Government of the Western Cape, August 2009. [ Links ]
7. Nkombua L. The practice of medicine at a district hospital emergency room: Middleburg Hospital, Mpumalanga Province. South African Journal of Family Practice 2009;50(1):65. [ Links ]
8. American Society of Anesthesiologists. Practice guidelines for sedation and analgesia by nonanaesthesiologists. Anaesthesiology 2002;96:1004-1017. [ Links ]
9. American College of Emergency Physicians. Clinical policy: procedural sedation and analgesia in the emergency department. Ann Emerg Med 2005;45:177-196. [ Links ]
10. American Academy of Pediatrics Committee on Drugs. Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures. Pediatrics 2002;110:836-839. [ Links ]
11. Mensour M, Pineau R, Sahai V, Michaud J. Emergency department procedural sedation and analgesia: A Canadian Community Effectiveness and Safety Study (ACCESS). CJEM 2006;8(2):94-99. [ Links ]
12. Roback MG, Wathen JE, Bajaj L, Bothner JP. Adverse events associated with procedural sedation and analgesia in a pediatric emergency department: A comparison of common parenteral drugs. Acad Emerg Med 2005;12(6):508-513. [ Links ]
13. Pena BM, Krauss B. Adverse events of procedural sedation and analgesia in a paediatric emergency department. Ann Emerg Med 1999;34(4Pt1):483-489. [ Links ]
14. Vardy JM, Dignon N, Mukherjee N, Sami DM, Balachandran G, Taylor S. Audit of the safety and effectiveness of ketamine for procedural sedation in the emergency department. Emerg Med J 2008;25:579-582. [ Links ]
15. British National Formulary (BNF) 46, September 2003. London: British Medical Association and Royal Phamaceutical Society of Great Britain, 2003. [ Links ]
16. Litman RS. Propofol and pediatric sedation (Letter). Ann Emerg Med 2004;44:184. [ Links ]
17. Bailey PL, Pace NL, Ashburn MA, Moll JW, East KA, Stanley TH. Frequent hypoxemia and apnea after sedation with midazolam and fentanyl. Anesthesiology 1990;73(5):826-830. [ Links ]
18. Agrawal D, Manzi SF, Gupta R, Krauss B. Preprocedural fasting state and adverse events in children undergoing procedural sedation and analgesia in a pediatric emergency department. Ann Emerg Med 2003;42:636-646. [ Links ]
19. Green SM. Fasting is a consideration - not a necessity - for emergency department procedural sedation and analgesia. Ann Emerg Med 2003;42:647-650. [ Links ]
20. Schwanda AE, Freyer DR, Sanfilippo DJ, et al. Brief unconscious sedation for painful pediatric oncology procedures: intravenous methohexital with appropriate monitoring is safe and effective. Am J Pediatr Hematol Oncol 1993;15:370-376. [ Links ]
21. Slonim AD, Ognibene FP. Sedation for pediatric procedures, using ketamine and midazolam, in a primarily adult intensive care unit: a retrospective evaluation. Crit Care Med 1998;26:1900-1904. [ Links ]
22. McCarver-May DG, Kang J, Aouthmany M, et al. Comparison of chloral hydrate and midazolam for sedation of neonates for neuroimaging studies. J Pediatr 1996;128:573-576. [ Links ]
23. Merola C, Albarracin C, Lebowitz P, et al. An audit of adverse events in children sedated with chloral hydrate or propofol during imaging studies. Paediatr Anaesth 1995;5:375-378. [ Links ]
Accepted 2 June 2011.
Corresponding author: G Wenzel-Smith (gisela@doctors.org.uk)














