Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.101 no.12 Pretoria Dez. 2011
ORIGINAL ARTICLES
Adolescent experiences in a vaccine trial: a pilot study
Amber AbramsI; Nandi SiegfriedII; Hennie GeldenhuysIII
IBA, MPhil. South African Cochrane Centre, Medical Research Council, Cape Town
IIMB ChB, MPH Hons, FCPHM (SA), PhD. Clinical Trials Support Initiative, Medical Research Council, Cape Town (currently: Department of Psychiatry, University of Cape Town)
IIIMB ChB, MMed (Fam Med) South African Tuberculosis Vaccine Initiative (SATVI), Institute of Infectious Diseases and Molecular Medicine, and School of Child and Adolescent Health, Faculty of Health Sciences, University of Cape Town
ABSTRACT
Little is known about how adolescents experience clinical trials. We assessed the experiences of South African adolescent participants in a clinical trial, employing semi-structured interviews to gather qualitative data on the experiences and effects of trial participation. Despite misunderstanding certain concepts regarding assent and trial processes subsequent to enrolment, participants reported positive experiences overall. Subjects' motivations for participation included: an ability to help others; receipt of healthcare; and free blood screening. Participants expressed fears associated with trial procedures, such as phlebotomy; however, these apprehensions diminished as the trial progressed. We found that conducting qualitative research within a trial site is feasible, and can provide insight into the uptake and acceptability of interventions.
Healthcare workers often practise dose extrapolation when prescribing medicines for children (< 18 years) owing to lack of data for this group.1 Incentives and legislation aimed at addressing this gap2 may increase the number of child-focused trials. However, there is little research on how this age group experiences clinical trials.3
Methods
We aimed to assess the experience of adolescent trial participants and evaluate the feasibility of conducting qualitative research within a clinical trial. The trial site was in the Breede Valley, Western Cape, where tuberculosis (TB) is endemic. Respondents were participants in a phase 1, randomised, placebo-controlled trial of a novel TB vaccine candidate.
We used a qualitative approach and conducted semi-structured interviews. Purposeful sampling was intended to ensure that expressed views reflected the diversity of the study population. Interviews were conducted in English with available interpretation in isiXhosa and Afrikaans. Interviews were subsequently transcribed, and translated into English by transcribers who were unaware of the research aims and participants' identities. We used manual thematic content analysis to identify emerging themes. Ethics committee approval of the study was obtained from the Medical Research Council and the University of Cape Town.
Results
After trial closure, 17 interviews had been conducted. Participants ranged from 13 to 18 years of age (median age 16). All participants spoke English at school. Home languages included Afrikaans (9 participants), isiXhosa (4), English (2) and a combination of languages (English/Afrikaans (1); English/isiXhosa (1)).
Most participants (9) reflected positively on trial participation; 3 described positive experiences despite instances of fear, e.g. 'It was very exciting ... when we're going along with the other children, we used to be scared ... but I really enjoyed it.' Two respondents conveyed a positive experience while reflecting on pain: 'It was nice ... but sometimes it was a little bit sore with the needles ... but ... you start to get used to it.'
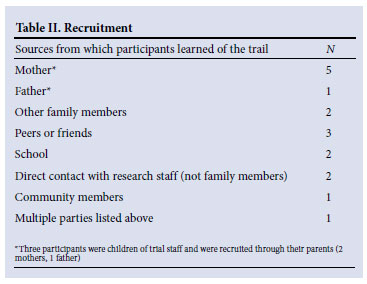
Participants varied in their knowledge of TB prior to the trial (Table I), and had learned of the trial from numerous sources (Table II). Fourteen participants discussed which person had assisted them with their decision to assent: 10 stated that a parent had helped them with their decision, 9 of whom said that this parent was their mother, and 2 stated that their mothers had enrolled them in the study.
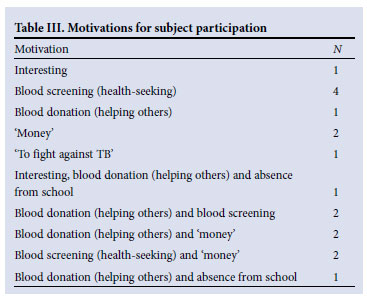
Motivations to participate were found to be multifactorial (Table III). The benefits of transport to the trial site, paired with altruistic urges to donate blood, were strong factors. Some participants hesitated to admit that 'money' motivated participation, but others were forthcoming: 'The money . . . helped us a lot, we even paid our burial society.' Health-seeking reasons were the most cited (8): 'It was an opportunity for me to know what my status is.' Altruistic urges (7) were important motivators: 'Mostly I didn't actually come for the money, I just came to get my blood statuses and to donate blood, mostly it was just donating my blood.'
Participants were prompted to reflect on the day before their first visit, and their overall emotions regarding trial participation; 7 described their excitement and usefulness owing to their involvement: 'I was excited and a little bit shy also ... It's not like every day they put a needle in your arm.' 'I felt useful for a change.' Five subjects reflected positively on the experience, stating that they felt comfortable; 3 remembered feeling scared initially, but, with increasing exposure to trial procedures, fears decreased. Although only 3 participants described feeling frightened, several revealed fears when discussing other aspects of participation: fears related to injections (6), the drawing of blood (3), blood test results (3) and anticipation (1)
Trial visits were an opportunity for subjects to connect with fellow community members, and to meet new people. All respondents made new connections at the trial site: 7 maintained contact while others (10) lost contact because the site visits ended.
Fifteen participants reported positive experiences with staff members, while highlighting the ability of the staff to amuse and explain procedures: 'They were easy to talk to, and can make you laugh at times. You get used to them. And then later on, they were like your friends.'
Participants differed in their perceptions of the purpose of the trial. Eleven thought that they understood the research, but only a few could explain the aim of the trial when asked to do so; 3 understood that the trial intended to test the efficacy of a TB vaccine ('It's to study if the vaccine was protecting you against TB'); 3 believed that the trial aimed to find an antidote to TB but did not expressly state that it was a vaccine trial; and 4 misunderstood the trial as blood screening for TB ('It was so that you could know if you were sick or - if you have TB'). Two participants understood the research as blood donation: 'I just know that, because they need more blood for other people.' Four participants stated that they did not know what the research aims were. Confusion between the drawing of blood (for screening) and blood donation was evident. Vaccine, injection and blood-drawing terminology were often used interchangeably. A minority (3) understood TB to be a sexually transmitted disease.
Eleven participants were able to recall the consent/assent process, 5 could not, and 1 reported that he/she was not present but that his/her mother was (in order for the formal consent process to be validated, the participant had to be present during a consenting session).
Perceived benefits that emerged when discussing the effect of the trial on participants' lives included increased awareness of TB (2), increased awareness of healthcare issues and places to access healthcare (2), overcoming the fear of needles/injections (2), access to a doctor (1), absence from school (1) and free blood screening (1). Participants stated that 'This place was good for me because I wouldn't have known if I had been ill', 'I didn't even know what TB was before I started ...', and 'I'm very afraid of needles, so I got over the fear of the sting.'
When asked for suggestions on how to improve their trial experience, 11 subjects stated that they would not change anything; only 1 reflected on the trial process: 'New technology so that stuff can go faster when they are taking your blood.'
All but 2 respondents answered that there were no negative aspects to their participation in the trial.
Discussion
We believe that this is the first South African study evaluating the experiences of adolescents in a TB vaccine trial. Overall, subjects' experiences were positive. Motivations to participate included: an ability to help others; receipt of healthcare; and free blood screening. Consistency of the employ of staff members whom participants knew from the community, and the provision of information by the former while maintaining a light mood were integral to participants' positive trial experiences. Qualitative research provided information-rich data that highlighted themes most salient to adolescent participants4 and the utility of such findings for informing trial research.
As with previous research,5 motivation to participate was driven primarily by altruism and health-seeking behaviour. Most subjects relied on their mothers' approval. Some suggested that their mothers had enrolled them in the trial, raising issues of whether assent can be withheld when parents have consented, especially where participation results in income for families.6 Context-based research is needed to understand the pressures that adolescents face in the decision-making process.
Participants recalled their fear of needles, blood collection and test results, which decreased with ongoing exposure. Trial participation, with pre-test counselling, could therefore reduce subjects' fears and other concerns.
The misunderstanding of the trial aims was not specific to the participants in this study. Misconceptions that trial participation protects participants from the researched disease need to be dispelled.7 Some participants were unaware that trial participation had prophylactic potential, instead believing that the purpose of the trial was for blood donation or screening.
Obtaining informed consent does not necessarily equate to informed participants. Limited recall of the informed consent process may indicate that less emphasis should be placed on a single informed consent session. Continuous consent,8 or repetition paired with discussion of trial details, may dispel confusion.
Strengths and limitations
The study strengths lie in trial participants being allowed the opportunity to voice their opinions. The time lapse of nearly a year between initial trial consent/assent and the subsequent interviews possibly limited recall. Interviews were conducted during a school holiday break which might have resulted in more positive responses, whereas those with negative experiences might have been less likely to attend interviews. Conducting interviews at the trial site might have introduced response bias. The use of interpreters during interviews might have led to loss of response nuances.
Further research is needed to understand adolescent consent/ assent experiences and their comprehension of the processes involved. This applies particularly to participant counselling in the context of limited funds. Ethnographic research should focus on understanding participation in a wider context.
Acknowledgements. We are grateful to the adolescents involved in this study, for their time and efforts. Special thanks to site staff for facilitation and support of the research. This study was fully funded by the MRC Clinical Trials Initiative and we are grateful to Dr Ali Dhansay for his support. Acknowledgements to Leon van Wyk for his services.
Author contributions. AA and NS developed the concept for the research. AA, NS and HG developed the interview guide, and AA conducted all interviews. All parties were involved in the synthesis and analysis of data. AA drafted this manuscript, and all authors contributed to the final version.
References
1. Conroy S, Choonara I, Impicciatore P, et al. Survey of unlicensed and off label drug use in paediatric wards in European countries. BMJ 2000;320(7227):79-82. [ Links ]
2. Greener M. Bitter medicine: New regulations aim to address the dearth of clinical safety trials for drugs used in children. Sci. Society 2008;9:505-508. [ Links ]
3. Read K, Fernandez CV, Gao J, et al. Decision-making by adolescents and parents of children with cancer regarding health research participation. Pediatrics 2009;124(3):959-965. [ Links ]
4. Donovan J, Mills N, Smith M, et al. Improving design and conduct of randomised trials by embedding them in qualitative research: ProtecT (prostate testing for cancer and treatment) study. BMJ 2002;325(7367):766-770. [ Links ]
5. Seelig BJ, Dobelle WH. Altruism and the volunteer: psychological benefits from participating as a research subject. ASAIO J 2001; 47(1):3-5. [ Links ]
6. Scherer DG, Annett RD, Brody JL. Ethical issues in adolescent and parent informed consent for pediatric asthma research participation. J Asthma 2007;44(7):489-496. [ Links ]
7. Simon AE, Wu AW, Lavori PW, Sugarman J. Preventive misconception: its nature, presence, and ethical implications for research. Am J Prev Med 2007;32(5):370-374. [ Links ]
8. Lema VM. Therapeutic misconception and clinical trials in sub-Saharan Africa: a review. East Afr Med J 2009;86(6):291-299. [ Links ]
Accepted 30 September 2011.
Corresponding author: A Abrams (aabrams@mrc.ac.za)














