Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.101 no.11 Pretoria Nov. 2011
ORIGINAL ARTICLES
Is non-therapeutic aspirin use in children a problem in South Africa?
Kirsten DonaldI; Susan HallII; Cylene SeatonIII; Donald TanyanyiwaIV
IMB ChB, MPhil (Paed Neurol), FCPaed (SA), DCH (SA), MRCPCH. Department of Paediatrics, School of Child and Adolescent Health, Red Cross War Memorial Children's Hospital, Cape Town
IIMB BS, BSc, FRCP, FFPHM, FRCPCH. Department of Paediatrics, School of Child and Adolescent Health, Red Cross War Memorial Children's Hospital, Cape Town
IIINational Diploma Medical Technology, National Higher Diploma Biochemistry. Chemistry Laboratory, Red CrossWar Memorial Children's Hospital
IVMB ChB, FIMLSc (Zimbabwe), FCPath (SA), MMedPath (SA). NHLS, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg
ABSTRACT
BACKGROUND: Aspirin should not be used in children except for specific therapeutic reasons. We report on a severely ill infant who had ingested aspirin contained in a traditional medicine and review 21 other patients with pre-admission non-therapeutic salicylate exposure.
OBJECTIVES AND METHODS: We reviewed laboratory, clinical and poisons unit records to determine how many children were admitted to our hospital over an 18-month period with evidence of salicylate ingestion not prescribed for therapeutic reasons. We determined the source of the salicylate, elapsed time between ingestion and laboratory assay, morbidity and mortality and final diagnosis.
RESULTS: Twenty-one children meeting our criteria, including 9 under 6 months of age, were admitted during this period. The most prevalent source of salicylate was over-the-counter (OTC) aspirin, but some had reportedly only been given traditional medicines. Nineteen were seriously ill, 4 died and 3 had severe brain injury. Two, initially diagnosed with Reye's syndrome, probably had inherited metabolic disorders. Only 2 patients had salicylate levels that at the time of measurement are normally considered toxic; however, the literature suggests that lower levels may exacerbate illness severity in young children.
CONCLUSIONS: We found inappropriate use of OTC aspirin in children that requires explanation. There may be policy implications for the content and presentation of patient information; the incorporation of pharmaceuticals in traditional medicines merits further study. Salicylate toxicity should be considered in children with unexplained metabolic acidosis out of keeping with the severity of their acute illness.
The dangers of aspirin use in children and young teenagers are well documented. In severely ill young children, aspirin can cause or exacerbate electrolyte and metabolic derangements as a result of the narrow dosage window between therapeutic and toxic effects.1-3 In industrialised countries, aspirin is not recommended in paediatric practice except for approved therapeutic purposes such as Kawasaki disease, because of its association with Reye's syndrome (RS)3 and the availability of equally effective antipyretic agents. Regulatory authorities in the UK, the USA and other industrialised countries have required warnings on all aspirin-containing preparations about their use in children and teenagers since the 1980s.4,5
Our study was prompted by a case of severe aspirin poisoning in an infant whose mother had unknowingly administered salicylate to her baby in a traditional medicine. This case is described and the following questions are addressed: (i) How many children were admitted to our hospital over a defined period with evidence of salicylate ingestion? (ii) Why was the salicylate given? (iii) Was the salicylate administered as over-the-counter (OTC) aspirin or other types of salicylate, or was it a component of traditional medicines? (iv) What were the morbidity and mortality rates of this group? (v) What are the clinical and public health implications of our findings?
Index case
A previously well 7-week-old male infant was admitted to Red Cross War Memorial Children's Hospital (RCH) with a 4-day history of poor feeding, cough and progressively rapid respiration. His mother had consulted a traditional healer who prescribed a liquid herbal mixture. This was given for 3 days prior to admission, but the infant's condition deteriorated. No other medications were reported. On admission the child was shocked and encephalopathic with fever, pallor, marked jaundice, and an acidotic breathing pattern. Admission investigations revealed hypoglycaemia, anaemia and urinary tract infection. Escherichia coli was later cultured from an admission urine specimen.
His mother brought the herbal mixture for analysis. It was in a clear unlabelled Coca Cola bottle and was green with a dense white sediment comprising 20 - 25% of the volume. A qualitative assessment of the salicylate content of the medicine indicated a 2+ level of salicylate. Independent analysis by a forensic laboratory confirmed the presence of salicylic acid. Salicylate was also detected in the patient's urine. The patient responded rapidly to treatment and was discharged well after 2 weeks with a final diagnosis of urinary tract infection complicated by salicylate poisoning.
Methods
Review of laboratory and clinical records
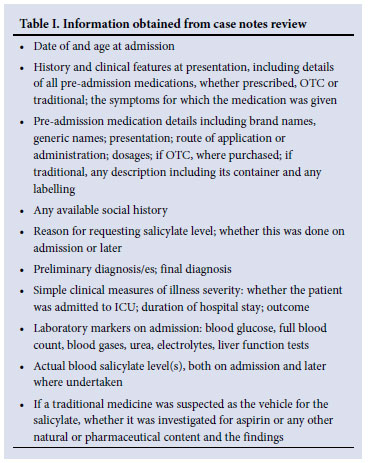
Clinical chemistry laboratory records between January 2006 and June 2007 were reviewed to identify all patients for whom blood salicylate levels had been requested. Those with a detectable level (defined by the hospital laboratory as 0.1 mmol/l) were further selected for case note review. A case was defined as any child presenting to RCH between January 2006 and June 2007 with a detectable blood salicylate level. Plasma salicylate was measured by fluorescence polarisation immunoassay technology (Abbott Laboratories). Information gathered on the cases is summarised in Table I. Patients taking aspirin for approved therapeutic reasons and having a blood level measured for monitoring purposes were excluded.
Poisons unit data
As a check on the completeness of case ascertainment, the poisons unit at RCH was asked to provide data on trends in discharges and deaths due to salicylate poisoning between January 2004 and December 2008. Cases were included on the unit's database if there had been a clinical diagnosis of salicylate poisoning based on a history of ingestion and suggestive clinical and laboratory features; confirmation by a documented blood salicylate level was not required.
Patient data handling
Data from laboratory records and case notes were anonymised, transcribed onto a study proforma and entered on an Excel spreadsheet. Each data field recorded whether that particular item was present, negative or missing. Permission to undertake the study was obtained from the University of Cape Town Ethics Committee, the Medical Superintendent, the Laboratory Director and the Director of the Poisons Unit at RCH.
Results
Laboratory and clinical record review
Salicylate levels were requested for a total of 156 patients over the 18-month review period: 25 had detectable levels, but case notes were unavailable for 5, leaving 20 available for review. The patients' ages ranged between 3 days and 6 years (median 8.5 months); 9 were aged under 6 months. There were 13 males and 7 females. Duration of admission ranged between 1 and 56 days (mean 15.7, median 10.5). Two patients were hospitalised for 2 months and 9 were admitted to the intensive care unit.
Ethnic and social background. Fifteen of the 20 patients' carers were Xhosa speaking, 1 was Afrikaans speaking and 1 was English speaking; this detail was not recorded in 3 cases. Social grading was available for 18 patients and was recorded as 'H1' in all 18. This compares with a prevalence of 77.7% of H1 grading among all patients seen in the emergency unit between January 2005 and June 2006, and of 68.9% among all patients admitted to acute paediatric general medicine in this period. H1 is the lowest category and equates to a family income <R4 800 per month (as stated at registration). The geographical distribution of most of the patients' homes reflected that of the poorest parts of Cape Town. The type of housing was recorded for 15 patients: 9 lived in shacks, 5 in a brick-built flat or house and 1 in a hostel.
Pre-admission clinical histories. The duration of illness, where recorded (N=15), ranged from 1 day to 2 months (median 7 days). The presence or absence of fever as a symptom in the history was recorded for 8 cases; it was present in 6 of these. Gastro-intestinal symptoms and/or respiratory symptoms were reported in 10 and 13 patients, respectively. Neurological symptoms were reported in 3 patients, of whom all had had seizures and one had also lost consciousness; one of these patients had had a closed head injury. One patient, otherwise well, presented with acute urticaria.
Overall pre-admission medications (Table II). A total of 16 of the 20 patients had a history of receiving medication: in 9 cases this was a pharmaceutical (8 being aspirin-containing preparations), and in 5 it was a traditional medicine. Two patients had been given both; the pharmaceuticals both contained aspirin. The reasons for giving preadmission medication were recorded for 10 cases; fever was the reason in only 4 cases. Other reasons included diarrhoea, cough, ear discharge, fits and 'fast breathing'. There was no clear difference between reasons for using traditional as opposed to OTC medications. In 1 case the administration of traditional medicines was initially denied by the mother and only elicited on further questioning the day after admission. Information on the source of these medicines was only provided for 1 case; it was a traditional healer.
Clinical features on admission. Although clinical data were often incomplete, all patients except one were extremely ill, with a range of clinical features and patterns: 8 were described as shocked and another as 'acutely ill'; 15 had one or more abnormal neurological findings reported (of whom 7 were encephalopathic, 2 had a decreased level of consciousness, 4 were fitting and 2 had meningism); and 10 patients had an acidotic breathing pattern. Table II shows the admission diagnoses - gastro-enteritis and sepsis predominated.
Laboratory findings. Table III summarises relevant data. Only 2 patients out of 12 who had a blood glucose measurement were hypoglycaemic; 6 children were hyponatraemic, 4 hypernatraemic, and 4 hypokalaemic. The urea was raised in 6, with urea/creatinine ratios suggesting a prerenal picture. Liver function was abnormal in 9 of 12 patients tested, but most cases were mild and in keeping with multisystem effects of sepsis in small infants; one of these had a confirmed diagnosis of hepatitis A. The plasma ammonia level was abnormal in 5 of 7 patients in whom it was measured.
Twelve of the patients were acidotic and 1 was alkalotic; of those who had normal pH values, an additional 4 had base excess values outside normal reference range, indicating compensated metabolic acidosis. Only 2 patients' salicylate levels fell into the toxic range defined by the laboratory (>2.2 mmol/l). However, for most of the 12 cases in which the information was recorded, the measurement was not undertaken until many hours after ingestion.
The reasons for requesting a salicylate level were recorded for 17 patients. The commonest (8 cases) was a history of ingestion of aspirin; in 3 others it was prompted by a history of ingestion of traditional medicines. For the remaining 6 patients it was because of clinical features on admission such as altered consciousness, acidotic breathing or acidosis not in keeping with the level of dehydration. It subsequently emerged that 4 of these 6 had a history of ingestion of aspirin or traditional medicine or both.
Outcome
Information on final diagnosis was available for 19 patients (Table II); 4 died and 3 survived with severe brain injury resulting in spastic quadriplegia with global delays. No significant sequelae were recorded at discharge or follow-up for the remaining 12, although 7 failed to attend follow-up appointments. The 2 patients with significantly elevated plasma ammonia levels (Table III) had a discharge diagnosis of probable or possible RS. The first, who survived with brain injury, was subsequently suspected, on the basis of a urinary organic acid profile, to have a mitochondrial disorder; the other, who died, had a urinary organic acid profile suggestive of long-chain acyl CoA dehydrogenase deficiency. Salicylate poisoning was the recorded final diagnosis in only 2 patients. In none was there a record of any discussions with the caregiver concerning the administration of either OTC or traditional medicines.
Poisons unit data
Six patients with salicylate poisoning were recorded between January 2004 and December 2008. Blood salicylate levels had been measured in 4. Only 1 extra case in the relevant time period was identified which had not been ascertained by our review of laboratory records, because a blood salicylate level had not been measured. In contrast, none of the cases ascertained in the laboratory review appeared on the poisons unit database.
Discussion
During the 18-month study period at least 21 children were admitted to RCH having been administered pre-admission aspirin which had not been prescribed for therapeutic reasons. This number is a minimum because there were 5 more with missing case notes and other cases may not have had a salicylate level measured. The most prevalent source was OTC aspirin, but some patients had reportedly only been given traditional medicines. Only in our index case, however, was there laboratory proof that the traditional medicine was the source. There was no evidence of toxic exposure to forms of salicylate other than aspirin. The medication had been given for a wide range of symptoms. All except 2 of the patients were seriously ill, 4 died and 3 were left with severe brain injury.
Although only 2 of our cases had admission salicylate levels above the 2.2 mmol/l that indicates toxicity by the laboratory reference range, this may not represent the true extent of the toxic effects of aspirin on the patients' illnesses. We cannot tell to what extent the salicylate affected outcome, but the literature suggests that it was likely to have been deleterious.
Buchanan reviewed his experience at Chris Hani Baragwanath Hospital of the effects of aspirin given to infants and young children with severe illness (in contrast with accidental overdose in a healthy subject).1 He emphasised that blood levels are an unreliable guide to toxicity, because the extent of the acid-base disturbance caused both by the aspirin and by the underlying illness will affect the amount of salicylate entering the cerebrospinal fluid (CSF) and brain. In children aged 1 - 4 years a metabolic acidosis (which favours such transfer) can be expected with aspirin toxicity; the respiratory alkalosis seen in adults does not develop until after the age of 12 years. Buchanan concluded that toxic levels of blood salicylate in young children can be as low as 15 mg/100 ml (1.09 mmol/l). In our study nearly half the patients were less than 6 months old and the oldest was 6 years old; there were 2 babies less than a week old. Metabolic acidosis was a consistent finding in the majority, even if partially compensated in some. The fact that in three-quarters of the patients who had liver function tests these were abnormal may reflect not only the underlying illness but also the effects of aspirin, which can cause raised hepatic enzymes.6 Owing to their young age, and in most cases severe systemic illness with associated metabolic disturbance, these children were therefore particularly vulnerable to the toxic effects of aspirin.
A study of the age-related pharmacokinetics of aspirin showed that infants have much lower elimination rates than 10 - 15-year-olds.7 Another important consideration is therefore the interval between ingestion and first measurement of salicylate level. For nearly half of our patients the time of ingestion was unknown or unrecorded; among the rest in only 3 cases was it probably less than 24 hours, and in 1 case the measurement was done at least 6 days later. Done8 showed that this is the most important variable affecting the relationship between the level and clinical signs of toxicity in patients with accidental aspirin poisoning. He developed a nomogram for retrospectively estimating the highest post-ingestion level based on a series of previously well subjects - the effects of age and illness were still unknown.
We therefore hypothesise that, regardless of observed blood levels, several patients in our series may have suffered salicylate toxicity which probably exacerbated their underlying illness. A study of children in Kenya,2 admitted with initial diagnoses of severe malaria, drew the same conclusion; 90% had detectable salicylate, some at toxic levels. The authors suggest that in some children salicylate poisoning may cause or contribute to the development of severe metabolic acidosis and hypoglycaemia, complications of malaria associated with high mortality. The biological plausibility of this clinical observation has subsequently been supported on theoretical grounds.9
The dangers of aspirin in African children described above1,2 are not a major issue in industrialised countries where, because of the association with RS, aspirin is no longer recommended for children.10 A UK national guideline on the management of fever in children recommended as antipyretics only paracetamol and ibuprofen.11 In the UK, labelling legislation passed in 1986 was modified in 2003, stating that aspirin should not be used in children under 16 years except on the advice of a doctor and that all OTC aspirin-containing preparations should carry warnings on the external packaging and on the patient information leaflet inside.4 Similar legislation has been promulgated in the USA.5 Following these measures, the incidence of RS declined dramatically in both countries.12,13
A diagnosis of RS was considered in 2 of our patients (Table II, cases 1 and 2) on the basis of liver function tests and, in case 2, on autopsy findings, but in both subsequent investigation suggested that a 'Reye-like' inherited metabolic disorder was more probable.14 Also, in one of these patients the salicylate level was still at 0.93 mmol/l on day 6 after admission, so it may have been at toxic levels earlier in the illness.8 Neither patient had the clinical features of 'classic' aspirin-associated RS, which rarely if ever occurs in young infants (our cases were aged 8 months and 3 days, respectively) and should only be diagnosed after other conditions have been excluded. Patients with RS-like features in this age group should first be exhaustively investigated for one of the many inherited metabolic disorders that mimic the condition before diagnosing RS.14
Our findings led us to speculate whether the use of OTC aspirin in children may be a problem in our area and elsewhere in South Africa, at least in poorer communities. Comorbidity caused by salicylate toxicity may be under-recognised. Among infants and children admitted to a hospital in the Eastern Cape with a diagnosis of 'herbal intoxication', 13.5% were recorded on admission to have acidotic breathing; 50% had respiratory distress. Nearly half died, mostly those aged <6 months. The investigation or possibility of salicylate toxicity was not reported or discussed.15

Our study shows that, in contrast to industrialised populations, some young children in the poorer areas of Cape Town are receiving aspirin. In addition to its adverse clinical effects, this raises several issues of public health significance. First, do the hazards associated with aspirin and its low cost justify its continued easy availability for symptomatic relief in childhood illnesses in South Africa? Second, what are the extent and effect of pharmaceuticals added to traditional medicines? Third, are parents sufficiently informed about the risks associated with aspirin use in their children, in OTC and traditional medicines? We will report elsewhere the information about aspirin use provided on packaging and package inserts, both of which are regulated by the Medicines Control Council. In resource-poor countries with low literacy levels, providing accessible information about the hazards of medicines will always be a challenge.
Acknowledgements. We acknowledge the team at RCH poisons unit (particularly Dr Kate Bloch) who gave help and time to answer queries and extract data from their database. We also thank Professor Sir David Hall (Honorary Professor, School of Child and Adolescent Health, University of Cape Town) for helpful editorial reviews of the manuscript.
References
1. Buchanan N. Salicylate intoxication in infancy: a review. S Afr Med J 1975;49:349-353. [ Links ]
2. English M, Marsh V, Amukoye E, Lowe B, Murphy S, Marsh K. Chronic salicylate poisoning and severe malaria. Lancet 1996;347:1736-1737. [ Links ]
3. Forsyth BW, Horwitz RI, Acampora D, et al. New epidemiologic evidence confirming that bias does not explain the aspirin/Reye syndrome association. JAMA 1989;261:2517-2524. [ Links ]
4. http://www.legislation.gov.uk/uksi/2003/1618/introduction/made (accessed 7 September 2011). [ Links ]
5. Federal Register/Vol. 68, No. 74/Thursday, April 17, 2003/Rules and Regulations pp. 18861-18869. http://frwebgate1.access.gpo.gov/cgi-bin/TEXTgate.cgi?WAISdocID=TC2kAO/1/1/0&WAISaction=retrieve (accessed 7 September 2011).
6. Rainsford KD. Side effects and toxicology. In: Rainsford KD, ed. Aspirin and Related Drugs. London & New York: Taylor & Francis, 2004:367-486. [ Links ]
7. Buchanec J, Galanda V, Visnovsky P, Halakova E. Effect of age on pharmacokinetics of salicylate. J Pediatr 1981;99:833-934. [ Links ]
8. Done AK. Salicylate intoxication: significance of measurements of salicylate in blood in cases of acute ingestion. Pediatrics 1960;26:800-807. [ Links ]
9. Clark I, Whitten R, Molyneux M, Taylor T. Salicylates, nitric oxide, malaria, and Reye's syndrome. Lancet 2001;357:625-627. [ Links ]
10. Paediatric Formulary Committee. British National Formulary for Children (2009). London: BMJ Publishing Group, RPS Publishing and RCPCH Publications, 2009. [ Links ]
11. www.nice.org.uk/CG047 (accessed 7 September 2011). [ Links ]
12. Hall SM, Lynn R. Reye's syndrome. N Engl J Med 1999;341:845-846. [ Links ]
13. Monto AS. The disappearance of Reye's syndrome - a public health triumph. N Engl J Med 1999;340:1423-1424. [ Links ]
14. Green A, Hall SM. Investigation of metabolic disorders resembling Reye's syndrome. Arch Dis Child 1992;67:1313-1317. [ Links ]
15. Tindimwebwa G, Dambisya YM. When is it herbal intoxication? A retrospective study of children admitted with herbal intoxication at Umtata General Hospital, South Africa. Cent Afr J Med 2003;49:111-114. [ Links ]
Accepted 30 August 2011.
Corresponding author: s.hall@sheffield.ac.uk)














