Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.101 no.11 Pretoria Nov. 2011
ORIGINAL ARTICLES
Tuberculosis in a South African prison : a transmission modelling analysis
Simon Johnstone-RobertsonI; Stephen D LawnII; Alex WelteIII; Linda-Gail BekkerIV; Robin WoodV
IBSc. Desmond Tutu HIV Centre, Institute of Infectious Diseases and Molecular Medicine, University of Cape Town, and DST/NRF Centre of Excellence in Epidemiological Modelling and Analysis, Stellenbosch University, Tygerberg, W Cape
IIBMedSci, MB BS, MRCP (UK), MD, DTM&H, Dip HIV Med. Desmond Tutu HIV Centre, Institute of Infectious Diseases and Molecular Medicine, University of Cape Town, and Department of Clinical Research Unit, Faculty of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine, London, UK
IIIPhD. DST/NRF Centre of Excellence in Epidemiological Modelling and Analysis, Stellenbosch University
IVMB BS, FCP (SA), PhD. Desmond Tutu HIV Centre, Institute of Infectious Diseases and Molecular Medicine, and Department of Medicine, University of Cape Town
VBSc, BM, BCh, DTM&H, MMed, FCP (SA). Desmond Tutu HIV Centre, Institute of Infectious Diseases and Molecular Medicine, University of Cape Town, DST/NRF Centre of Excellence in Epidemiological Modelling and Analysis, Stellenbosch University, and Department of Medicine, University of Cape Town
ABSTRACT
BACKGROUND: Prisons are recognised internationally as institutions with very high tuberculosis (TB) burdens where transmission is predominantly determined by contact between infectious and susceptible prisoners. A recent South African court case described the conditions under which prisoners awaiting trial were kept. With the use of these data, a mathematical model was developed to explore the interactions between incarceration conditions and TB control measures.
METHODS: Cell dimensions, cell occupancy, lock-up time, TB incidence and treatment delays were derived from court evidence and judicial reports. Using the Wells-Riley equation and probability analyses of contact between prisoners, we estimated the current TB transmission probability within prison cells, and estimated transmission probabilities of improved levels of case finding in combination with implementation of national and international minimum standards for incarceration.
RESULTS: Levels of overcrowding (230%) in communal cells and poor TB case finding result in annual TB transmission risks of 90% per annum. Implementing current national or international cell occupancy recommendations would reduce TB transmission probabilities by 30% and 50%, respectively. Improved passive case finding, modest ventilation increase or decreased lock-up time would minimally impact on transmission if introduced individually. However, active case finding together with implementation of minimum national and international standards of incarceration could reduce transmission by 50% and 94%, respectively.
CONCLUSIONS: Current conditions of detention for awaitingtrial prisoners are highly conducive for spread of drug-sensitive and drug-resistant TB. Combinations of simple well-established scientific control measures should be implemented urgently.
Prisons have high burdens of tuberculosis (TB) where overcrowding, lack of ventilation and poor prevention practices dramatically increase transmission risks of TB.1-5 The TB burden is exacerbated in sub-Saharan Africa by a high prevalence of HIV infection among inmates, as TB is the most common opportunistic infection among people living with HIV in Africa.6 A high TB prevalence and poor control policies within prisons also create potential breeding grounds for multidrug-resistant TB (MDR-TB).4 TB transmission within prisons can also significantly impact on the wider community.5
South Africa has the fourth highest global incarceration rate, with more than 165 000 prisoners in 237 operational prisons.7 There is a rapid turnover of awaiting-trial prisoners with 79% being incarcerated for periods of less than 12 months.8 The number of individuals passing through the prison system annually therefore exceeds 368 000.8 Detainees either awaiting sentencing or awaiting trial comprise approximately a third of prisoners; they suffer the worst prison conditions, frequently being kept in large crowded communal cells housing 40 -60 inmates for 23 hours per day.7-10 International agencies recommend a minimum allocation of 5.4 m2 of floor space per prison inmate,11 while South African prison regulations stipulate a minimum allocation of 3.34 m2 floor area in communal cells.7 However, awaiting-trial prisoners are frequently housed in overcrowded communal cells for prolonged periods of time with floor space allocations as low as 1.4 m2 per inmate.7-10
South African prisons' TB notifications have not been reported in the public domain or included in the annual judicial prison inspectorate reports.7-10 The consequences of overcrowding on TB transmission in prisons have therefore not previously been quantified. However, previous incarceration was found to be a significant risk factor for prevalent TB in a population survey in Cape Town.12 Prison inmates elsewhere have been identified as at high risk for TB, including MDR-TB and extensively drug-resistant TB (XDR-TB).4-6
A judgment was published in the case of Dudley Lee and the Minister of Correctional Services in which the plaintiff developed TB while an awaiting-trial prisoner in Pollsmoor prison, Cape Town.13 Evidence during the trial described an understaffed and poorly functioning prison TB control programme, and data were presented on TB incidence, delays in accessing TB diagnosis and care, hours of lock-up, crowding and poor ventilation.
We aimed to explore probabilities of TB transmission of awaitingtrial prisoners incarcerated in communal cells in Pollsmoor correctional facility, Cape Town. We used the court case evidence13 as parameters in a deterministic model14,15 of the risks of TB transmission during imprisonment.
Methods
Study design
TB transmission probabilities were estimated using the Wells-Riley equation, a well-known transmission model that has been applied to a wide range of transmission scenarios,14-20 including describing airborne transmission probabilities within a single enclosed room or space with defined ventilation characteristics.15 We used this equation in combination with the distribution of inmates per cell and their probability of having TB that had been infectious for different periods of time, in order to explore prisoner-to-prisoner TB transmission probabilities. The modelled transmission probabilities were adjusted for daily lock-up periods and variable cell ventilation characteristics.
We then explored the effects of decreased crowding, shorter lock-up times, improved ventilation and improved case finding on TB transmission probabilities. Finally we explored combinations of changes to the TB control programme and prison conditions necessary to achieve significant reductions in TB transmission. Table I shows the values and ranges of key parameters used to populate the model.
Prison population
Pollsmoor maximum-security prison is the third-largest facility housing unsentenced prisoners in South Africa, with approximately 3 200 awaiting-trial and unsentenced prisoners at any time. They are predominantly incarcerated in communal cells of 40 - 60 prisoners and confined for 23 hours each day.13 Overcrowding is persistently high with reported average occupancy rates of 235% in 200313 and 239% in 2008.7 Cell ventilation is poor with a single slatted window on an exterior wall with openings of 6 088 cm2 and a small ventilator grille with area of 126 cm2 on the solid metal door, which is closed at night.
The TB control programme
South African prisons' TB control programme is similar to the national TB programme, which focuses on passive case finding of sputum smear-positive cases and directly observed short-course therapy.25 However, because of chronic nursing shortages the strategy was poorly implemented, with no active case finding, and inmates with symptomatic TB could wait up to 4 months before referral to the prison hospital.13 Medical staff did not systematically screen newly arriving prisoners for symptoms or signs of TB.13 Notification registers between 1998 and 2009 were inconsistently completed, resulting in significant under-reporting of TB cases; 177 prisoners commenced TB therapy in 2001 - a notification rate of 5.5 TB cases per 100-person prison years.13 However, a prison medical officer gave evidence that during the year, 264 prisoners had laboratory confirmation of acid-fast bacilli on direct sputum smear, indicating marked under-reporting of a TB incidence rate of 8.25 cases per 100-person prison years, that MDR-TB was prevalent among inmates, and that a staff member had died from this form of the disease.13
Mathematical transmission model
The number of TB infections (C) occurring in a prison cell with susceptible prisoners (S) was assumed to be a function of the number of infectious cases (I), their infectivity (q = quanta of infectious particles produced per hour), time of exposure (t = time of exposure in minutes), respiration rate (p = litres per hour), and germ-free ventilation (Q = litres per hour) as given by Wells-Riley equation C = S(1-e-Iptq/Q). The prevalence (P) of infectious adults at any time is given by the annual smear-positive incidence rate (M = per cent) and the period of infectivity (Δ = days) as P = M/[365/ Δ]. The risk of contact with an infectious adult is given by the Poisson distribution (λI/I!)e-λ, where λ; = P*(A-1) is the expected number of infectious cases in a cell with 'A = I + S' adults.
Modelled input parameters
Germ-free ventilation (Q) was calculated as air changes per hour (ACH) for a standard cell of 9.1 m long × 6.4 m wide × 3.35 m high with a volume of 195 m3. Current cell ventilation would provide less than 1 ACH with all windows and the door ventilator grille open with totally free flow of air through the cell and a 10 km/h wind directed towards the window.26 International recommendations for prison ventilation22 based on the floor area of this cell would recommend 1.8 -3.58 ACH, and the World Health Organization (WHO) recommends 12 ACH for health settings and congregate settings where TB is prevalent.23 Four values of ACH were therefore modelled: the status quo of 1 ACH (poor ventilation); 3 ACH (minimum international recommended ventilation); 8 ACH (intermediate ventilation); and 12 ACH (optimal ventilation).
A wide range of estimated values for the rate of production of infectious TB quanta (q) have been reported. Laryngeal TB is highly infectious with 'q' estimated at 60 infectious quanta per hour.16 In a workplace outbreak due to an untreated smear-positive pulmonary case, 'q' was estimated at 12.7 infectious quanta per hour.18 Over a 2-year period in a TB ward, 'q' was directly measured at 1.25 infectious quanta per hour.15 A study applying molecular strain characterisation to track airborne TB transmission from HIV/TB-infected inpatients to guinea pigs demonstrated markedly variable infectiousness.27 Values of 'q' for infectious cases varied between 3 and 12 and 2.5 and 226 quanta per hour for individuals with drug-sensitive and MDR-TB respectively. In order to be conservative, 'q' was modelled at a mean value of 1 infectious quantum per hour.
The mean respiratory rate of adults (p) was estimated to be 360 litres per hour corresponding to a normal adult respiratory minute volume of 6 litres per minute.24
A key parameter of the model, the period of infectiousness (Δ), has a strong inverse association with the TB control programme effectiveness of case finding. Δ is a composite of delays, including time to access medical care, diagnostic delay and time to commence chemotherapy. The diagnostic delay period during which an adult may be infective is variable, but is frequently reported to be 60 - 90 days.21 However, delays in accessing treatment within this prison were reported to be very prolonged; therefore analyses were performed with values of Δ from 1 day up to 180 days.
Passive case finding depends on individuals with symptoms of TB self-presenting for investigation. It was modelled that with increased TB awareness health messaging, minimal delay in accessing TB services, expeditious diagnosis and rapid initiation of chemotherapy, the period of infectiousness (Δ) could be reduced to 60 days. Active case finding (regular seeking out symptomatic prisoners) and rapid diagnostic testing28 was modelled with values of Δ of less than 60 days.
Results
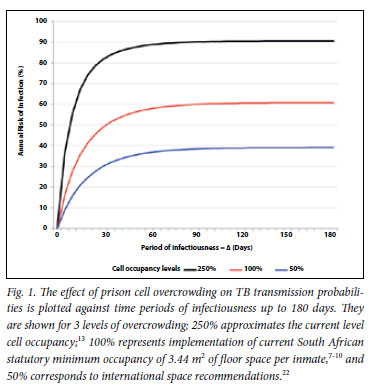
We explored the impact of cell occupancy on TB transmission probabilities. Transmission probabilities at existing levels of overcrowding, the recommended minimum South African and international recommended occupancy are shown across a spectrum of time periods of infectiousness of source cases (Δ) from 1 to 180 days in Fig. 1. Transmission probabilities under prevailing conditions of incarceration were estimated at 90% per annum for all values of Δ (>60 days) currently implementable by the prison TB control programme. The benefits of decreasing cell crowding were proportionate at all values of Δ. Implementing current South African recommended cell minimum levels of occupancy would reduce transmission by 30% and implementing international recommendations would reduce transmission by over 50%, even with current levels of TB case finding.
The effect of decreasing lock-up time (period restricted to cells each day) is shown for the existing conditions of 23 hours per day and for reductions to 12 and 8 hours per day respectively in Fig. 2. The benefits of decreasing lock-up time are modest at current values of Δ (approximately 180 days). However, the benefits of decreased lockup times are amplified by improving case finding with consequent reductions in Δ. When Δ is reduced to 60 days, reduction of lock-up time to 12 and 8 hours would reduce TB transmission by 10% and 20%, respectively.

The effect of cell ventilation on TB transmission probability is shown in Fig. 3. Three levels of ventilation were modelled in addition to the current reported estimate of 1 ACH: 3 ACH; 8 ACH; and 12 ACH. Improved ventilation markedly decreases TB transmission probabilities at all values for Δ. However, improvements in ventilation are amplified when accompanied by reductions in the value of Δ which could be achieved by improved case finding.

Finally, we explored effects of improved case finding in three different scenarios (Fig. 4): scenario 1 - status quo; scenario 2 - current South African regulations for imprisonment with modest increase of ventilation to 3 ACH fully implemented; and scenario 3 - international standards for imprisonment together with ventilation at 12 ACH fully implemented. Improving passive case finding to achieve a Δ of 60 days would have minimal effect on TB transmission in scenario 1 and 20% and 50% reductions in scenarios 2 and 3, respectively. Active case finding to achieve a Δ of 30 days would decrease transmission by 10% with current scenario 1, 50% with implementation of scenario 2, and 90% with implementation of scenario 3.

Discussion
This study shows that conditions prevailing in a South African prison are extremely conducive for ongoing transmission of TB. Crowding, substandard living conditions and a poorly functioning prison TB control programme combine to contribute to high TB transmission risks. Overcrowding of cells directly and proportionately increases the probability of contact with infectious sources. Delays of 3 - 4 months in accessing medical care,13 together with time required for diagnosis and implementing therapy,21 markedly increase the prevalence of infectious cases, and act as the primary source for ongoing transmission.
We show that the very high prevalence of infectious cases within the prison population potentially negates the benefits of improved ventilation and shortened exposure time within cells. The interdependence of all the transmission risk factor parameters is further highlighted by the observation that improved passive case finding sufficient to reduce the period of infectiousness from 6 to 2 months would have a minimal effect under current conditions of crowding and poor ventilation. However, the multiplicative benefits of concurrent improvements in case finding, crowding and environmental conditions are demonstrated. Active case finding and implementing current national minimum standards of cell occupancy7 can reduce transmission by 50%. Introducing international environmental standards22, 23 could reduce transmission from the status quo by as much as 94%.
Our study strength was available accurate information specific to this prison: number of TB cases, cell dimensions, number of prisoners per cell and likely delays in accessing TB treatment. A limitation of the model is that precise enumeration of the number of infective quanta produced by a prisoner with TB is difficult. A very conservative estimate was therefore derived from published data.15-20,25,26 The modelled analysis was also restricted to transmission events within an illustrative typical communal prison cell within a single operational prison. However, similar and even more severely crowded conditions are endemic in South African prisons.7-10 The model was based on the epidemiological assumption that the TB epidemic was generalised, with equal mixing of infectivity and contact risks. Therefore the analysis was restricted to transmission events occurring within the cell, and stochastic transmission events such as close contact with highly infectious individuals outside the cell are not captured. Despite these limitations, the model outputs are compatible with the very high TB incidence rate relative to the rest of Cape Town's population,13,29 and robustly demonstrated the proportional impact of different control strategies on transmission probabilities.
The analysis was restricted to acquisition of infection and not development of disease. The relationship between newly acquired infection and disease development is complex.30,31 Prior TB infection in immune-competent individuals, which is common in Cape Town,32 gives some protection against disease related to subsequent infection.33 However, those not previously infected,34 those with previously treated TB31 and those with HIV infection35 would be particularly vulnerable to progress to active TB disease following recent exposure to infection. We did not specifically address transmission of MDR-and XDR-TB, but transmission risks with these may be heightened as a result of the prolonged period of infectiousness that often results from failure of diagnosis and subsequent receipt of inappropriate therapy. However, accurate MDR- and XDR-TB prevalence data are not available to permit modelling.
'A society should be judged not by how it treats its outstanding citizens but by how it treats its criminals.'36 We show that the conditions in which awaiting-trial prisoners are confined fall far below our own national7-10,13 and international standards for incarceration22,23 and constitute a health emergency. The medical and environmental health professions and the judiciary must urgently work with the Department of Correctional Services to institute simple scientifically based disease control measures that need to be tailored to circumstances and resources. Many strategies are available to address the problem. The Judicial Inspectorate has repeatedly proposed the measures required to decrease the awaiting-trial prison population.7-10 Cell ventilator grills should not be closed at night; cross-ventilation of communal cells could be encouraged by using barred rather than solid doors and incorporating corridor ventilator extraction systems. Since 1847, carbon dioxide levels have been used as a measure of adequate ventilation37 and carbon dioxide monitoring could readily establish if effective improvements in cell ventilation are being achieved. Prison TB control programmes should introduce active case finding21 and use recent technological advances in rapid TB diagnosis and drug resistance.28 TB notification data for South African prisons should not be considered secret or restricted information, but accurate data should be made available to the Judicial Inspectorate of Prisons to include in the annual report on the state of our prisons.7-10
TB transmission risks within our prison system are unacceptably high, posing a direct hazard to prisoners and contributing to the general population TB burden. Overlooking TB prevention and control in prisons carries serious health consequences for both prisoners and the general community.1-2,38
Acknowledgments. Dr Steven Craven, for providing information on Pollsmoor Prison conditions; former Visiting Principal Medical Officer, Pollsmoor Prison and Honorary Lecturer, Department of Family Medicine, University of Cape Town.
Financial support. National Institutes of Health (RO1 grant A1058736- 01A1 to RW and L-GB); USAID grant CA674A00080000700 (RW); Wellcome Trust, London, UK (SDL).
References
1. Van Niekerk JP. Lock up and stay: South Africa's sick prisons. S Afr Med J 2005;95(5):281. [ Links ]
2. Baussano I, Williams BG, Nunn P, et al. Tuberculosis incidence in prisons: A systematic review. PLoS Med 2010;7(12):e1000381. doi:10.1371/journal.pmed.100038 [ Links ]
3. Aerts A, Hauer B, Wanlin M, Veen J. Tuberculosis and tuberculosis control in European prisons. Int J Tuberc Lung Dis 2006;10:1215-1223. [ Links ]
4. O'Grady J, Mwaba P, Bates M, Kapata N, Zumla A. Tuberculosis in prisons in sub-Saharan Africa - a potential time bomb. S Afr Med J 2011;101(2):107-108. [ Links ]
5. Basu S, Stuckler D, McKee M. Addressing institutional amplifiers in the dynamics and control of tuberculosis epidemics. Am J Trop Med Hyg 2011;84(1):30-37. [ Links ]
6. HIV and prisons in sub-Saharan Africa: opportunities for action. UNAIDS, 2007. http://data.unaids.org/pub/Report/2007/hiv_prison_paper_en.pdf (accessed 6 September 2011). [ Links ]
7. Annual Report 2008/9. Treatment of inmates and conditions in correctional centres. Judge DH van Zyl, Judicial Inspectorate of Prisons. http://judicialinsp.pwv.gov.za (accessed 20 April 2011). [ Links ]
8. Annual Report for the period 1 April 2006 to 31 March 2007 of the Inspecting Judge of Prisons, Judge NC Erasmus. http://judicialinsp.pwv.gov.za (accessed 20 April 2011). [ Links ]
9. Annual Report for the period 1 April 2007 to 31 March 2008 of the Inspecting Judge of Prisons, Judge NJ Yekiso. Available at http://judicialinsp.pwv.gov.za (accessed 20 April 2011). [ Links ]
10. Annual Report for the period 1 April 2005 to 31 March 2006 of the Inspecting Judge of Prisons, Judge J Fagan. http://judicialinsp.pwv.gov.za (accessed 20 April 2011). [ Links ]
11. Water, Sanitation, Hygiene and Habitat in Prisons. August 2005. International Committee of the Red Cross. Geneva, Switzerland: Red Cross, 2005. [ Links ]
12. Wood R, Middelkoop K, Myer L, et al. The burden of undiagnosed tuberculosis in an African community with high HIV-prevalence: implications for TB control. Am J Respir Crit Care Med 2007;175:87-93. [ Links ]
13. Lee v Minister of Correctional Services, Case No. 10416/04. Judgment in the matter between Dudley Lee and The Minister of Correctional Services. Western Cape High Court, Cape Town, South Africa. http://www.saflii.org/za/cases/ZAWCHC/2011/13.html (accessed 20 April 2011). [ Links ]
14. Wells WF. Airborne contagion and air hygiene. Cambridge, MA: Harvard University Press, 1955. [ Links ]
15. Riley Rl, Wells WF, Mills CC, Nyka W, McLean RL. Air hygiene in tuberculosis: quantative studies of infectivity and control in a pilot ward. Am Rev Tuberc 1957;75:420-431. [ Links ]
16. Cantazaro A. Nosocomial tuberculosis. Am Rev Respir Dis 1982;125:559-562. [ Links ]
17. Noakes CJ, Sleigh PA. Mathematical models for assessing the role of airflow on the risk of airborne infection in hospital wards. J R Soc Interface 2009;6:S791-800. [ Links ]
18. Nardell EA, Keegan J, Cheney SA, Etkind SC. Airborne infection: theoretical limits of protection achievable by building ventilation. Am Rev Respir Dis 1991;144:302-306. [ Links ]
19. Furuya H, Nagamine M, Watanabe T. Use of a mathematical model to estimate tuberculosis transmission risk in an Internet cafe. Environ Health Prev Med 2009;14:96-102. [ Links ]
20. Wood R, Johnstone-Robertson S, Uys P, et al. Tuberculosis transmission to young children in a South African community: modelling household and community infection risks. Clin Infect Dis 2010:51(4):401-408. [ Links ]
21. Storla DG, Yimer S, Bjune GA. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health 2008;8:1-9. [ Links ]
22. Dara M, Grzemska M, Kimerling ME, Reyes H, Zagorskiy A. 2009 Guidelines for Control of Tuberculosis in Prisons. Tuberculosis Coalition for Technical Assistance and international committee of the Red Cross. Geneva: Red Cross, 2009. [ Links ]
23. World Health Organization. WHO policy on TB infection control in health-care facilities, congregate settings and households. Geneva: World Health Organization, 2009. http://whqlibdoc.who.int/publications/2009/9789241598323_eng.pdf (accessed 16 February 2010). [ Links ]
24. Pinna GD, Maestri R, La Rovere MT, Gobbi E, Fanfulla F. Effect of paced breathing on ventilatory and cardiovascular variability parameters during short-term investigations of autonomic function. Am J Physiol Heart Circ Physiol 2006;290(1):H424-33. [ Links ]
25. Department of Health, South Africa. The South African Tuberculosis Control Programme: practical guidelines 2000. http://www.capegateway.gov.za/Text/2003/tb_guidelines2000.pdf (accessed 6 September 2011). [ Links ]
26. Lindament MW. A guide to energy efficient ventilation. Air Infiltration and Ventilation Centre, International Energy Agency, 1996. http://www.aivc.org/ (accessed 13 April 2010). [ Links ]
27. Escombe AR, Moore DAJ, Gilman RH, et al. The infectiousness of tuberculosis patients coinfected with HIV. Plos Med 2008;9:e188. doi:10.137/journal.pmed.0050188 [ Links ]
28. Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 2010:363:1005-1015. [ Links ]
29. Wood R, Lawn SD, Johnstone-Robertson S, Bekker L-G. Tuberculosis control has failed in South Africa: time to reappraise strategy. S Afr Med J 2011;101(2):111-114. [ Links ]
30. Reider HL. Epidemiological basis of tuberculosis control. Paris: International Union against Tuberculosis and Lung Disease, 1999. [ Links ]
31. Opie E, McPhedran FM, Putnam P. The fate of persons in contact with tuberculosis: the exogenous infection of children and adults. Am J Epidemiol 1935;22:644-682. [ Links ]
32. Wood R, Liang H, Wu H, et al. Changing prevalence of TB infection with increasing age in high TB burden townships in South Africa. Int J Tuberc Lung Dis 2010;14:406-412. [ Links ]
33. Heimbeck J. Immunity to tuberculosis. Arch Intern Med 1928;41:336-342. [ Links ]
34. Bjartveit K. Olaf Scheel and Johannes Heimbeck: their contribution to understanding the pathogenesis and prevention of tuberculosis. Int J Tuberc Lung Dis 2003;7(4):306-311. [ Links ]
35. Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study Lancet 2002;359:2059-2064. [ Links ]
36. Dostoevsky F. Crime and Punishment. Oxford University Press, 1998. [ Links ]
37. De Chaumont F. On the theory of ventilation: an attempt to establish a positive basis for the calculation of the amount of fresh air required for an inhabited air-space. Proc R Soc Lond 1874;23:187-201. [ Links ]
38. Reyes H, Coninx R. Pitfalls of tuberculosis programmes in prisons. BMJ 1997;315:1447-1450. [ Links ]
Accepted 2 June 2011.
All authors confirm no potential conflicts of interest.
Corresponding author: Linda-Gail Bekker (robin.wood@hiv-research.org.za)














