Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SAMJ: South African Medical Journal
versión On-line ISSN 2078-5135
versión impresa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.101 no.10 Pretoria oct. 2011
SCIENTIFIC LETTERS
Clinically significant anaerobic bacteria isolated from patients in a South African academic hospital : antimicrobial susceptibility testing
S NaidooI; O PerovicII; G A RichardsIV; A G DuseIII
IMSc (Med). Department of Clinical Microbiology and Infectious Diseases, School of Pathology of the National Health Laboratory Service, and University of the Witwatersrand, Johannesburg
IIMD, DTM&H, FCPath (SA) Microbiol, MMed (Microbiol). Department of Clinical Microbiology and Infectious Diseases, School of Pathology of the National Health Laboratory Service, and University of the Witwatersrand, Johannesburg
IIIMB BCh, DTM&H, MMed (Microbiol), MScMed, FCPath (SA) Microbiol. Department of Clinical Microbiology and Infectious Diseases, School of Pathology of the National Health Laboratory Service, and University of the Witwatersrand, Johannesburg
IVMB BCh, PhD, FCP (SA). Department of Critical Care, Charlotte Maxeke Johannesburg Academic Hospital and University of the Witwatersrand
ABSTRACT
BACKGROUND: Increasing resistance to some antimicrobial agents among anaerobic bacteria has made susceptibility patterns less predictable.
METHOD: This was a prospective study of the susceptibility data of anaerobic organisms isolated from clinical specimens from patients with suspected anaerobic infections from June 2005 until February 2007. Specimens were submitted to the microbiology laboratory at Charlotte Maxeke Johannesburg Academic Hospital, where microscopy, culture and susceptibility testing were performed the using E test® strip minimum inhibitory concentration method. Results were interpreted with reference to Clinical and Laboratory Standards Institute guidelines for amoxicillin-clavulanate, clindamycin, metronidazole, penicillin, ertapenem, cefoxitin, ceftriaxone, chloramphenicol and piperacillin-tazobactam.
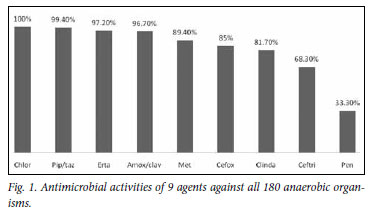
RESULTS: One hundred and eighty anaerobic isolates were submitted from 165 patients. The most active antimicrobial agents were chloramphenicol (100% susceptible), ertapenem (97.2%), piperacillin-tazobactam (99.4%) and amoxicillin-clavulanic acid (96.7%). Less active were metronidazole (89.4%), cefoxitin (85%), clindamycin (81.7%), ceftriaxone (68.3%) and penicillin (33.3%).
CONCLUSION: Susceptibility testing should be performed periodically to identify emerging trends in resistance and to modify empirical treatment of anaerobic infections.
To the Editor: Anaerobic bacteria (anaerobes) are the predominant flora on human skin and mucous membranes and are a common cause of endogenous infections. Anaerobes are commonly found in polymicrobial infections in combination with aerobes, and in this setting therapy should be directed towards both types of pathogens. Antibiotic resistance among anaerobes has increased, and antibiotics that were reliably effective, such as metronidazole, are no longer as active.1 Since culture of anaerobes is not within the scope of many laboratories, susceptibility testing is not routinely performed.
We prospectively studied antibiotic susceptibility profiles of anaerobes isolated from clinical specimens routinely tested in the microbiology laboratory at Charlotte Maxeke Johannesburg Academic Hospital (CMJAH) from June 2005 until February 2007. Our objectives were to determine the antimicrobial susceptibility patterns of anaerobes isolated from clinical specimens, initiate a surveillance programme to monitor the susceptibility profiles of anaerobes, and identify their changing trends in antibiotic susceptibility and resistance.
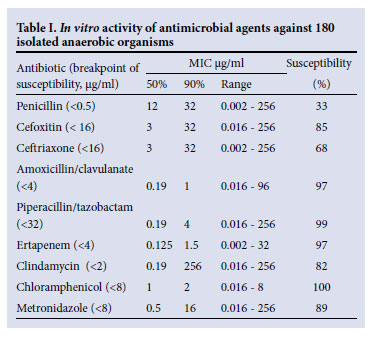
Specimens from patients with suspected mixed aerobic/anaerobic infections were submitted in anaerobic transport media to the microbiology laboratory, where microscopy, culture and susceptibility testing to amoxicillin-clavulanate, clindamycin, metronidazole, benzylpenicillin, ertapenem, cefoxitin, ceftriaxone, chloramphenicol and piperacillin-tazobactam were performed. Antimicrobial susceptibility testing to record the minimum inhibitory concentration (MIC) profile was performed on all isolates using the E test® strip method according to the manufacturer's instructions. Interpretation of the MIC was performed using the Clinical and Laboratory Standards Institute (CLSI) guidelines.2
Quality control was performed with organisms of known susceptibility. Control strains used were Bacteroides fragilis ATCC 25285, B. thetaiotaomicron ATCC 29741 and Eubacterium lentum ATCC 43055. All isolates were processed according to standard operating procedures (SOPs) established at the CMJAH Microbiology Laboratory. Anaerobes were identified using the Finegold system, which included selective growth media, biochemical profiles and susceptibility to antimicrobial agents, and by the use of rapid ID 32A API panels.12 Statistical analysis was performed using the WHONET 5.4 programme.
Anaerobes were submitted from 165 patients (139 adults, 18 children, 8 unknown age); all 180 anaerobes were identified to species level, with B. fragilis being the most common. Most specimens were submitted from surgical wards (29%), emergency room (17%), general ICU (10%) and gynaecology (6%).
The most active agents against these organisms were chloramphenicol (100% of isolates susceptible), ertapenem (97.2%), piperacillin-tazobactam (99.4%) and amoxicillin-clavulanate (96.7%). Less active were metronidazole (89.4%), cefoxitin (85%), clindamycin (81.7%), ceftriaxone (68.3%) and penicillin (33.3%) (Fig. 1). Table I presents the MIC50 and MIC90 and the susceptibility ranges.
Organisms within the B. fragilis group were isolated from 97 patients (54%), comprising B. fragilis (81), B. thetaiotaomicron, (4), B. distasonis (1), B. ovatus (5), B. vulgatus (3), B. eggerthii (1), B. uniformis (1) and Bacteroides spp. (1). Overall this group demonstrated 13.4% resistance to metronidazole.
Anaerobic organisms such as B. fragilis 81 (45%), Clostridium perfringens 23 (13%), Peptostreptococcus anaerobius 14 (8%) and Prevotella melaninogenica 14 (8%) were analysed separately. Chloramphenicol, piperacillin-tazobactam, ertapenem and amoxicillin-clavulanate demonstrated the highest activity against
these isolates, while 11% and 18% demonstrated resistance to metronidazole and clindamycin, respectively.
Discussion
Our study illustrates the dynamic changes in antimicrobial susceptibility that have occurred among anaerobes and emphasises a decrease in antimicrobial susceptibility compared with a survey in Cape Town in 1995.8 Of particular concern is the prevalence of metronidazole resistance that is largely unrecognised by clinicians. Susceptibility profiles of B. fragilis were similar to those from Brazil,4 demonstrating resistance rates of 12% for cefoxitin, 15.1% for cefotaxime, 1% for chloramphenicol, 18.2% for clindamycin, 75.7% for tetracycline and 16% for metronidazole. In our study, resistance to cefoxitin was 8.6%, to clindamycin 14.8% and to metronidazole 12.3%, but there was no resistance to chloramphenicol or amoxicillin-clavulanate. Others5,6 have reported clindamycin resistance rates as high as 33% in B. fragilis, and 36%, 49% and 46% in B. thetaiotamicron, B. distasonis and B. caccae, respectively. Oteo et al.7 reported an overall resistance rate of 49% to clindamycin for the B. fragilis group, while in our study resistance to clindamycin was 18.7%.
A Cape Town study8 demonstrated that 4% (total 26) of C. perfringens isolates were resistant to benzylpenicillin and clindamycin, but all were sensitive to cefoxitin, metronidazole, chloramphenicol and amoxicillin-clavulanate. C. perfringens, C. fallax and C. sordelli in this study exhibited no resistance to penicillin or metronidazole, while the single isolate of C. septicum showed highlevel resistance to metronidazole (MIC 256 µg/ml).The single isolate of C. paraputrificum was resistant to clindamycin (MIC 128 µg/ml) and cefoxitin (MIC 256 µg/ml).
P. melaninogenica (15 isolates) were resistant to penicillin and metronidazole in 60% and 6.7%, respectively. Two isolates of Veillonella parvula were resistant to penicillin.
Among all isolated anaerobic organisms, 97.2% were susceptible to ertapenem. This was similar to the findings of Goldstein et al.,9 who demonstrated that ertapenem was consistently active against the B. fragilis group, but not against 12 (20%) of strains of Bilophila wadsworthia, 3 (5%) lactobacilli, and 1 Acidaminococcus fermentans.
Pfister prospectively investigated 370 clinical isolates of anaerobic bacteria over 6 months.10 With the exception of one isolate of Fusobacterium varium and B. fragilis (MIC 32 µg/ml), all were also sensitive to ertapenem.
In our study, among 20 strains of peptostreptococci, 35% were resistant to penicillin and 5% to clindamycin, with no resistance to metronidazole. In contrast, in Koch et al.'s study,8 10% of 20 strains of P. anaerobius were resistant to benzylpenicillin, cefoxitin and metronidazole; in addition Peptostreptococcus spp. (total of 17) showed resistance to benzylpenicillin in 12% and to metronidazole and clindamycin in 6%, respectively.
Appelbaum and Chatterton11 in 1978, in a study similar to that in South Africa on 265 anaerobic bacteria from clinical isolates, found low levels of resistance to penicillin, chloramphenicol, clindamycin and metronidazole. However, in our study 100% remain susceptible to chloramphenicol, but only 82% to clindamycin, 89% to metronidazole and 33% to penicillin.
Conclusion
We demonstrated a worrying increase in resistance to metronidazole, particularly in the B. fragilis group, and highlight the high rates of intermediate susceptibility to other anti-anaerobic agents. This emphasises the necessity for periodic active surveillance to identify and record these emerging trends.
Acknowledgements. The authors wish to thank to all technologists from the Microbiology Laboratory at Charlotte Maxeke Johannesburg Academic Hospital for their assistance.
Funding. MIC testing by E test was provided by an International MSD Research Grant.
Ethics. This study was approved by Human Research Ethics Committee (Medical) at the University of the Witwatersrand.
References
1. Meyer L, Brink D, Weldhagen GF. Antibiotic susceptibility patterns of anaerobic bacteria isolated in Pretoria, South Africa, during 2003-2004. Southern African Journal of Epidemiology and Infection 2006;21(4):161-163. [ Links ]
2. Clinical Laboratory Standards Institute. Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria, Approved Standards. 6th ed. 2005, M11-A6, Vol 24, No 2. Clinical and Laboratory Standards Institute, Pennsylvania, USA [ Links ]
3. Wybo I, Pierard D, Verschraegen I, et al. Third Belgian multicentre survey of antibiotic susceptibility of anaerobic bacteria. J Antimicrob Chemother 2007;59:132-139. [ Links ]
4. Paula GR, Falcao LS, Antunes ENF, et al. Determinants of resistance in Bacteroides fragilis strains according to recent Brazilian profiles of antimicrobial susceptibility. International Journal of Antimicrobial Agents 2004;24:53-58. [ Links ]
5. Bandoh K, Veno K, Watanabe K, et al. Susceptibility patterns and resistance to imipenem in the Bacteroides fragilis group species in Japan: a 4-year study. Clin Infect Dis 1993;16(Suppl 4):S382-S386. [ Links ]
6. Betriu C, Gomez M, Palau ML, et al. Activities of new antimicrobial agents (trovafloxacin, moxifloxacin, sanfetrinem, quinupristin-dalfopristin) against Bacteroides fragilis group: comparison with the activities of 14 other agents. Antimicrob Agents Chemother 1999;43:2320-2322. [ Links ]
7. Oteo J, Aracil B, Alo's JI, et al. High prevalence of resistance to clindamycin in Bacteroides fragilis group isolates. J Antimicrob Chemother 2000;45:691-693. [ Links ]
8. Koch CL, Derby P, Abratt VR. In-vitro antibiotic susceptibility and molecular analysis of anaerobic bacteria in Cape Town, South Africa. J Antimicrob Chemother 1998;42:245-248 [ Links ]
9. Goldstein EJC, Citron Diane M, MerriamVreni C, et al. Comparative in vitro activities of ertapenem (MK-0826) against 1,001 anaerobes isolated from human intraabdominal infections. J Antimicrob Chemother 2000;44(9):2389-2394. [ Links ]
10. Pfister W. In vitro activity of ertapenem in comparison with penicillin, piperacillin/tazobactam, clindamycin, and metronidazole against clinical isolates of anaerobic bacteria. 14th European Congress of Clinical Microbiology and Infectious Diseases, 1-4 May 2004, Prague. Abstract No. 902, p. 1887. [ Links ]
11. Appelbaum PC, Chatterton SA. Susceptibility of anaerobic bacteria to ten antimicrobial agents. Antimicrob Agents Chemother 1978;14:371-376. [ Links ]
Accepted 5 August 2011.
Corresponding author: O Perovic (olga.perovic@nhls.ac.za, olgap@nicd.ac.za)














